| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 2,2,3,3-Tetramethylbutane | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.008.961 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| UN number | 1325 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H18 |
| Molar mass | 114.232 g·mol |
| Appearance | White, opaque, waxy crystals |
| Odor | Odorless |
| Melting point | 98 to 104 °C; 208 to 219 °F; 371 to 377 K |
| Boiling point | 106.0 to 107.0 °C; 222.7 to 224.5 °F; 379.1 to 380.1 K |
| Henry's law constant (kH) |
2.9 nmol Pa kg |
| Thermochemistry | |
| Heat capacity (C) | 232.2 J K mol (at 2.8 °C) |
| Std molar entropy (S298) |
273.76 J K mol |
| Std enthalpy of formation (ΔfH298) |
−270.3 – −267.9 kJ mol |
| Std enthalpy of combustion (ΔcH298) |
−5.4526 – −5.4504 MJ mol |
| Hazards | |
| GHS labelling: | |
| Pictograms |    
|
| Signal word | Danger |
| Hazard statements | H228, H304, H315, H336, H410 |
| Precautionary statements | P210, P240, P241, P261, P264, P271, P273, P280, P301+P310, P302+P352, P304+P340, P312, P321, P331, P332+P313, P362, P370+P378, P391, P403+P233, P405, P501 |
| Flash point | 4 °C (39 °F; 277 K) |
| Explosive limits | 1–?% |
| Related compounds | |
| Related alkanes | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
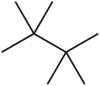
Tetramethylbutane, sometimes called hexamethylethane, is a hydrocarbon with formula C8H18 or (H3C-)3C-C(-CH3)3. It is the most heavily branched and most compact of the octane isomers, the only one with a butane (C4) backbone. Because of its highly symmetrical structure, it has a very high melting point and a short liquid range; in fact, it is the smallest saturated acyclic hydrocarbon that appears as a solid at a room temperature of 25 °C. (Among cyclic hydrocarbons, cubane, C8H8 is even smaller and is also solid at room temperature.) It is also the most stable C8H18 isomer, with a heat of formation 4.18 kcal/mol (17.5 kJ/mol) lower than that of n-octane, a fact that has been attributed to stabilizing dispersive interactions (electron correlation) between the methyl groups (protobranching).
The compound can be obtained from ethyl bromide, tert-butyl bromide, and magnesium metal in the presence of manganese(II) ions. Despite conditions amenable to the formation of a Grignard reagent, organomagnesium compounds are believed not the active species. Instead, they transmetalate to organomanganese compounds, which then decompose to tert-butyl radicals, which dimerize.
The full IUPAC name of the compound is 2,2,3,3-tetramethylbutane, but the numbers are superfluous in this case because there is no other possible arrangement of "tetramethylbutane".
References
- "Hexamethylethane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Information. Retrieved 11 March 2012.
- Joyce, Justin P.; Shores, Matthew P.; Rappè, Anthony K. (2020-07-29). "Protobranching as repulsion-induced attraction: a prototype for geminal stabilization". Physical Chemistry Chemical Physics. 22 (29): 16998–17006. Bibcode:2020PCCP...2216998J. doi:10.1039/D0CP02193H. ISSN 1463-9084. PMID 32676632. S2CID 220606477.
- M. S. KHARASCH; J. W. HANCOCK; W. NUDENBERG; P. O. TAWNEY (1956). "Factors Influencing the Course and Mechanism of Grignard Reactions. XXII. The Reaction of Grignard Reagents with Alkyl Halides and Ketones in the Presence of Manganous Salts". Journal of Organic Chemistry. 21 (3): 322–327. doi:10.1021/jo01109a016.