| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.007.337 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C24H20AsCl |
| Molar mass | 418.80 g·mol |
| Appearance | white solid |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H301, H331, H410 |
| Precautionary statements | P261, P264, P270, P271, P273, P301+P310, P304+P340, P311, P321, P330, P391, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
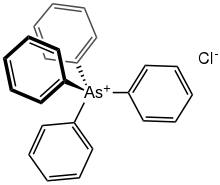
Tetraphenylarsonium chloride is the organoarsenic compound with the formula (C6H5)4AsCl. This white solid is the chloride salt of the tetraphenylarsonium cation, which is tetrahedral. Typical of related quat salts, it is soluble in polar organic solvents. It often is used as a hydrate.
Synthesis and reactions
It is prepared by neutralization of tetraphenylarsonium chloride hydrochloride, which is produced from triphenylarsine:
- (C6H5)3As + Br2 → (C6H5)3AsBr2
- (C6H5)3AsBr2 + H2O → (C6H5)3AsO + 2 HBr
- (C6H5)3AsO + C6H5MgBr → (C6H5)4AsOMgBr
- (C6H5)4AsOMgBr + 3 HCl → (C6H5)4AsClHCl + MgBrCl
- (C6H5)4AsClHCl + NaOH → (C6H5)4AsCl + NaCl + H2O
Like other quat salts, it is used to solubilize polyatomic anions in organic media. To this end, aqueous or methanolic solutions containing the anion of interest are treated with a solution of tetraphenylarsonium chloride, typically resulting in precipitation of the tetraphenylarsonium anion salt.
Related compounds
References
- Shriner, R. L.; Wolf, Calvin N. (1950). "Tetraphenylarsonium Chloride Hydrochloride". Organic Syntheses. 30: 95. doi:10.15227/orgsyn.030.0095.
- Dieck, R. L.; Peterson, E. J.; Galliart, A.; Brown, T. M.; Moeller, T. (1976). "Tetraethylammonium, Tetraphenylarsonium, and Ammonium Cyanates and Cyanides". Inorganic Syntheses. Vol. 16. pp. 131–137. doi:10.1002/9780470132470.ch36. ISBN 9780470132470.