| |

| |
| Names | |
|---|---|
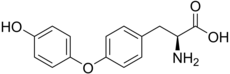
| IUPAC name O-(4-Hydroxyphenyl)-L-tyrosine | |
| Systematic IUPAC name (2S)-2-Amino-3-propanoic acid | |
| Other names 4-(4-Hydroxyphenoxy)-L-phenylalanine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.014.986 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C15H15NO4 |
| Molar mass | 273.28 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Thyronine is a metabolite derived from thyroxine and triiodothyronine via the peripheral enzymatic removal of iodines from the thyroxine nucleus. Thyronine is the thyroxine nucleus devoid of its four iodine atoms.
References
- Pubchem Compound summary, L-thyronine
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |
| Thyroid hormone metabolic intermediates | |
|---|---|
| Tyrosine / iodotyrosine | |
| Thyronine / iodothyronine | |
| Thyronamine / iodothyronamine | |
| Iodothyroacetate / iodothyroacetic acid | |