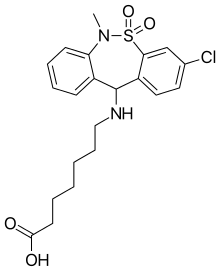
 Tianeptine Tianeptine | |
 Naloxone Naloxone | |
| Combination of | |
|---|---|
| Tianeptine | Atypical μ-opioid receptor agonist |
| Naloxone | Orally inactive μ-opioid receptor antagonist |
| Clinical data | |
| Other names | Naloxone/tianeptine; Tianeptine oxalate/naloxone; Naloxone/tianeptine oxalate; Tianeptine hemioxalate/naloxone; Naloxone/tianeptine hemioxalate; TNX-601; TNX601; TNX-601 CR; TNX-601-CR; TNX-601 ER; TNX-601-ER |
| Routes of administration | Oral |
Tianeptine/naloxone (developmental code names TNX-601, TNX-601-CR, TNX-601-ER), or naloxone/tianeptine, is an extended-release combination of tianeptine, an atypical μ-opioid receptor agonist, and naloxone, an orally inactive μ-opioid receptor antagonist, which was under development for the treatment of major depressive disorder, post-traumatic stress disorder (PTSD), and neurocognitive dysfunction associated with corticosteroid use but was never marketed.
Whereas tianeptine is marketed widely throughout Europe, Asia, and Latin America but is not available in the United States or the United Kingdom, tianeptine/naloxone was under development for registration in the United States and other countries. In addition, whereas tianeptine has a short duration of action and requires administration three times per day, tianeptine/naloxone was developed as an extended-release formulation with enhanced pharmacokinetics suitable for once-daily administration. The combination formulation employs tianeptine as the oxalate salt, which is said to have improved physicochemical properties for use in the extended-release formulation compared to the amorphous tianeptine sodium that is used in immediate-release tianeptine-only formulations. Naloxone is used in misuse-resistant oral drug formulations as it is inactive if taken orally but becomes active if oral tablets are crushed and administered parenterally, such as by injection.
Tianeptine/naloxone reached phase 2 clinical trials for major depressive disorder and phase 1 clinical trials for post-traumatic stress disorders and cognition dysfunction related to corticosteroid use prior to the discontinuation of its development. Its development was discontinued for all indications in October 2023 due to lack of effectiveness for major depressive disorder in a phase 2 clinical trial.
References
- ^ "Naloxone/tianeptine - Tonix Pharmaceuticals". AdisInsight. 2 November 2023. Retrieved 21 October 2024.
- ^ "Delving into the Latest Updates on Tianeptine Oxalate(Tonix Pharmaceuticals, Inc.) with Synapse". Synapse. 19 September 2024. Retrieved 21 October 2024.
- ^ "CNS Summit 2021: Abstracts of Poster Presentations: TNX-601 CR*: A ONCE-DAILY FORMULATION OF TIANEPTINE IN DEVELOPMENT FOR THE TREATMENT OF MAJOR DEPRESSIVE DISORDER IN THE UNITED STATES". Innovations in Clinical Neuroscience. 18 (10-12 Suppl 1): S5 – S16. 2021. PMC 8752122.
- ^ Sullivan GM, Hsu DT, Peters A, Peters P, Fogarty S, Kiu R, Meibohm B, Lederman S. TNX-601 CR*: a Once-Daily Formulation of Tianeptine in Development for the Treatment of Major Depressive Disorder (PDF). CNS Summit 2021, Nov 7-10, 2021.
- "Tonix Pharmaceuticals Announces Development of TNX-601 ER, a Potential Abuse Deterrent, Extended-Release Formulation of Tianeptine Oxalate for the Treatment of Major Depressive Disorder". Tonix Pharmaceuticals Holding Corp. 11 July 2022. Retrieved 21 October 2024.
- Pergolizzi JV, Raffa RB, Taylor R, Vacalis S (April 2018). "Abuse-deterrent opioids: an update on current approaches and considerations". Curr Med Res Opin. 34 (4): 711–723. doi:10.1080/03007995.2017.1419171. PMID 29262730.
- "Tonix Pharmaceuticals Announces Topline Results from Phase 2 Proof-of-Concept Study of TNX-601 ER for the Treatment of Major Depressive Disorder". BioSpace. 31 October 2023. Retrieved 21 October 2024.