Tm is an abbreviation for anionic tridentate ligand based on three imidazole-2-thioketone groups bonded to a borohydride center. They are examples of scorpionate ligands. Various ligands in this family are known, differing in what substituents are on the imidazoles. The most common is Tm, which has a methyl group on the nitrogen. It is easily prepared by the reaction of molten methimazole (1-methylimidazole-2-thione) with sodium borohydride, giving the sodium salt of the ligand. Salts of the Tm anion are known also for lithium and potassium. Other alkyl- and aryl-group variations are likewise named Tm according to those groups.

Ligand characteristics, comparison with Tp
The Tm anion is a tridentate, tripodal ligand topologically similar to the more common Tp ligands, but the two classes of ligands differ in several ways. Tm has three "soft" sulfur donor atoms, whereas Tp has three nitrogen donor atoms. The thioamide sulfur is highly basic, as found for other thioureas. The Tm anion simulates the environment provided by three facial thiolate ligands but without the 3- charge of a facial trithiolate.
The large 8-membered SCNBNCSM chelate rings in M(Tm) complexes are more flexible than the 6-membered CNBNCM rings in M(Tp) complexes. This flexibility enables the formation of boron-metal bonds, after loss of the B-H bond. This degradation of the coordinated Tm anion gives a dehydrogenated boranamide B(mt)3 where mt = methimazolate.
- Representative Structures (S in yellow, B in dark blue)
-
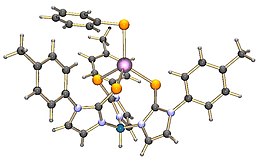 Structure of TmZnSPh, a tetrahedral complex.
Structure of TmZnSPh, a tetrahedral complex.
-
 Structure of TmRu(CO)(PPh3)H, an octahedral complex.
Structure of TmRu(CO)(PPh3)H, an octahedral complex.
References
- Mark D. Spicer, John Reglinski "Soft Scorpionate Ligands Based on Imidazole-2-thione Donors" European Journal of Inorganic Chemistry 2009, Issue 12, pp 1553–1574. doi:10.1002/ejic.200801240
- "Synthesis and Characterization of Novel Mononuclear Cadmium Thiolate Complexes in a Sulfur-Rich Environment" S. Bakbak, C. D. Incarvito, A. L. Rheingold, D. Rabinovich, Inorg. Chem., 2002, volume 41, p. 998. doi:10.1021/ic0109243
- "Polyazolyl Chelate Chemistry. 13. An Osmaboratrane" M. R. St. J. Foreman, A. F. Hill, A. J. P. White, and D. J. Williams Organometallics, 2004, volume 23, p. 913. doi:10.1021/om0306123. "The Sting of the Scorpion: A Metallaboratrane" A. F. Hill, G. R. Owen, A. J. P. White, and D. J. Williams Angew. Chem. Int. Ed. Engl., 1999, volume 38, p. 2759. doi:10.1002/(SICI)1521-3773(19990917)38:18<2759::AID-ANIE2759>3.0.CO;2-P