| |
| Names | |
|---|---|
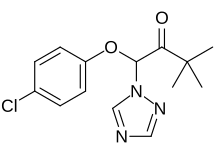
| IUPAC name 1-(4-Chlorophenoxy)-3,3-dimethyl-1-(1H-1,2,4-triazol-1-yl)butan-2-one | |
| Other names Triadimeform | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.050.986 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C14H16ClN3O2 |
| Molar mass | 293.75 g·mol |
| Density | 1.22 g/cm |
| Melting point | 82 °C (180 °F; 355 K) |
| Boiling point | decomposes |
| Solubility in water | 64 mg/L (20 °C) |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 363 mg/kg (oral, rat) > 5000 mg/kg (dermal, rat) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Triadimefon is a fungicide used in agriculture to control various fungal diseases. As a seed treatment, it is used on barley, corn, cotton, oats, rye, sorghum, and wheat. In fruit it is used on pineapple and banana. Non-food uses include pine seedlings, Christmas trees, turf, ornamental plants, and landscaping.
References
- ^ Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ "Triadimefon Reregistration Eligibility Decision (RED) and Triadimenol Tolerance Reassessment and Risk Management Decision (TRED) Fact Sheet". Environmental Protection Agency.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |