| |
| Names | |
|---|---|
| Preferred IUPAC name 1,1,2-Trifluoro-2-iodoethene | |
| Other names 1,1,2-Trifluoro-2-iodoethylene, trifluoroiodoethylene, iodotrifluoroethylene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.006.028 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
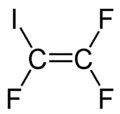
| Chemical formula | C2F3I |
| Molar mass | 207.92 g/mol |
| Density | 2.284 g/cm |
| Boiling point | 30 °C (86 °F; 303 K) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Irritant (Xi) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Iodotrifluoroethylene is the organofluorine compound with the formula C
2F
3I. It is a volatile colorless liquid.
Preparation and reactions
It is prepared by iodination of trifluorovinyl lithium.
Iodotrifluoroethylene reacts with cadmium metal to give CdC2F3(I).
It reacts with nitric oxide under UV light, producing a nitroso compound, with iodine as a byproduct:
- 2 C
2F
3I + 2 NO → 2 C
2F
3NO + I
2
References
- Burdon, James; Coe, Paul L.; Haslock, Iain B.; Powell, Richard L. (1996). "The hydrofluorocarbon 1,1,1,2-tetrafluoroethane (HFC-134a) as a ready source of trifluorovinyllithium". Chemical Communications: 49. doi:10.1039/CC9960000049.
- Burton, Donald J.; Yang, Zhen-Yu; Morken, Peter A. (1994). "Fluorinated organometallics: Vinyl, Alkynyl, Allyl, Benzyl, Propargyl and Aryl". Tetrahedron. 50 (10): 2993–3063. doi:10.1016/S0040-4020(01)81105-4.
- Griffin, C. E.; Haszeldine, R. N . (1960). "Perfluoroalkyl derivatives of nitrogen. Part VIII. Trifluoronitrosoethylene and its polymers". Journal of the Chemical Society (Resumed): 1398–1406. doi:10.1039/JR9600001398.
This article about an alkene is a stub. You can help Misplaced Pages by expanding it. |
This article about an organic halide is a stub. You can help Misplaced Pages by expanding it. |