For the flame retardant chemical, see tris(2-chloroethyl) phosphate.

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 3,3′,3′′-Phosphanetriyltripropanoic acid | |
| Other names
TCEP Tris(2-carboxyethyl)phosphine | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C9H15O6P |
| Molar mass | 250.187 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
TCEP (tris(2-carboxyethyl)phosphine) is a reducing agent frequently used in biochemistry and molecular biology applications. It is often prepared and used as a hydrochloride salt (TCEP-HCl) with a molecular weight of 286.65 gram/mol. It is soluble in water and available as a stabilized solution at neutral pH and immobilized onto an agarose support to facilitate removal of the reducing agent.
Synthesis
TCEP can be prepared by the acid hydrolysis of tris(cyanoethyl)phosphine.
Applications
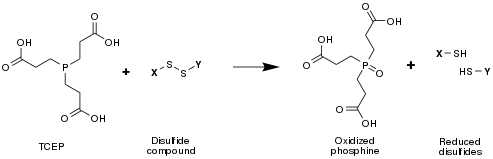
TCEP is often used as a reducing agent to break disulfide bonds within and between proteins as a preparatory step for gel electrophoresis.
Compared to the other two most common agents used for this purpose (dithiothreitol and β-mercaptoethanol), TCEP has the advantages of being odorless, a more powerful reducing agent, an irreversible reducing agent (in the sense that TCEP does not regenerate—the end product of TCEP-mediated disulfide cleavage is in fact two free thiols/cysteines), more hydrophilic, and more resistant to oxidation in air. It also does not reduce metals used in immobilized metal affinity chromatography.
TCEP is particularly useful when labeling cysteine residues with maleimides. TCEP can keep the cysteines from forming di-sulfide bonds and, unlike dithiothreitol and β-mercaptoethanol, it will not react as readily with the maleimide. However, TCEP has been reported to react with maleimide under certain conditions.
TCEP is also used in the tissue homogenization process for RNA isolation.
For Ultraviolet–visible spectroscopy applications, TCEP is useful when it is important to avoid interfering absorbance from 250 to 285 nanometers which can occur with dithiothreitol. Dithiothreitol will slowly over time absorb more and more light in this spectrum as various redox reactions occur.
History
Reduction of biomolecules with trialkyphosphines received little attention for decades because historically available phosphines were extremely malodorous and/or insoluble in water. In 1969, TCEP was reported as an oderless and water-soluble trialkyphosphine suitable for biochemical use, however the potential use of TCEP for biochemical applications was almost totally ignored for decades. In 1991, Burns reported a new convenient synthetic procedure for TCEP, which set off TCEP becoming more widely available and marketed as a "new" reducing agent for biochemical use, & thus TCEP came into more widespread use throughout the 1990s.
Reactions
TCEPT will reduce disulfides to thiols in the presence of water:
Via a similar process it can also reduce sulfoxides and N-oxides. Some other side reactions have also been reported:
- Conversion of a cysteine residue into alanine in the presence of TCEP and heat (90˚C).
- Slow (but significant, 40% cleavage reported for two week storage at 4˚C) protein backbone cleavage at cysteine residues under mild conditions.
Use in biological research
TCEP is available from various chemical suppliers as the hydrochloride salt. When dissolved in water, TCEP-HCl is acidic. A reported preparation is a 0.5 M TCEP-HCl aqueous stock solution that is pH adjusted to near-neutral pH and stored frozen at -20˚C. TCEP is reportedly less stable in phosphate buffers.
See also
- 2-Mercaptoethanol (BME)
- Dithiothreitol (DTT)
- Dithiobutylamine (DTBA)
References
- Yost JM, Knight JD, Coltart DM (15 September 2008). "Tris(2-carboxyethyl)phosphine Hydrochloride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00973.
- ^ TCEP technical information, from Interchim
- Shafer, D. E., Inman, J. K., Lees, A. (2002). "Reaction of Tris(2-carboxyethyl)phosphine (TCEP) with Maleimide and α-Haloacyl Groups: Anomalous Elution of TCEP by Gel Filtration". Anal. Biochem. 282 (1): 161–164. doi:10.1006/abio.2000.4609. PMID 10860517. S2CID 37825047.
- Tyagarajan K, Pretzer E, Wiktorowicz JE (2003). "Thiol-reactive dyes for fluorescence labeling of proteomic samples". Electrophoresis. 24 (14): 2348–2358. doi:10.1002/elps.200305478. PMID 12874870. S2CID 20446141.
- Rhee SS, Burke DH (2004). "Tris(2-carboxyethyl)phosphine stabilization of RNA: comparison with dithiothreitol for use with nucleic acid and thiophosphoryl chemistry". Anal. Biochem. 325 (1): 137–143. doi:10.1016/j.ab.2003.10.019. PMID 14715294.
- ^ Han J, Han G (1994). "A Procedure for Quantitative Determination of Tris(2-Carboxyethyl)phosphine, an Odorless Reducing Agent More Stable and Effective Than Dithiothreitol". Analytical Biochemistry. 220 (1). Elsevier BV: 5–10. doi:10.1006/abio.1994.1290. ISSN 0003-2697. PMID 7978256.
- Levison ME, Josephson AS, Kirschenbaum DM (1969). "Reduction of biological substances by water-soluble phosphines: Gamma-globulin (IgG)". Experientia. 25 (2). Springer Science and Business Media LLC: 126–127. doi:10.1007/bf01899076. ISSN 0014-4754. PMID 4182166. S2CID 20548859.
- Burns JA, Butler JC, Moran J, Whitesides GM (1991). "Selective reduction of disulfides by tris(2-carboxyethyl)phosphine". The Journal of Organic Chemistry. 56 (8). American Chemical Society (ACS): 2648–2650. doi:10.1021/jo00008a014. ISSN 0022-3263.
- Faucher AM, Grand-Maître C (October 2003). "tris (2-Carboxyethyl)phosphine (TCEP) for the Reduction of Sulfoxides, Sulfonylchlorides, N -Oxides, and Azides". Synthetic Communications. 33 (20): 3503–3511. doi:10.1081/SCC-120024730.
- Wang Z, Rejtar T, Zhou ZS, Karger BL (2010-01-04). "Desulfurization of cysteine-containing peptides resulting from sample preparation for protein characterization by mass spectrometry". Rapid Communications in Mass Spectrometry. 24 (3). Wiley: 267–275. doi:10.1002/rcm.4383. ISSN 0951-4198. PMC 2908508. PMID 20049891.
- Liu P, O'Mara BW, Warrack BM, Wu W, Huang Y, Zhang Y, Zhao R, Lin M, Ackerman MS, Hocknell PK, Chen G, Tao L, Rieble S, Wang J, Wang-Iverson DB, Tymiak AA, Grace MJ, Russell RJ (2010-01-28). "A tris (2-carboxyethyl) phosphine (TCEP) related cleavage on cysteine-containing proteins". Journal of the American Society for Mass Spectrometry. 21 (5). American Chemical Society (ACS): 837–844. doi:10.1016/j.jasms.2010.01.016. ISSN 1044-0305. PMID 20189823.
- ^ "Strategies for protein purification". Cytiva. Retrieved 24 February 2023.