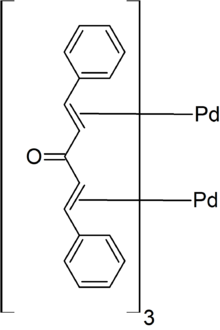
| |

| |

| |
| Names | |
|---|---|
| IUPAC name Tris(dibenzylideneacetone)dipalladium | |
| Other names Pd2(dba)3 | |
| Identifiers | |
| CAS Number | |
| ECHA InfoCard | 100.122.794 |
| PubChem CID | |
| Properties | |
| Chemical formula | C51H42O3Pd2 |
| Molar mass | 915.73 g·mol |
| Melting point | 152 to 155 °C (306 to 311 °F; 425 to 428 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Tris(dibenzylideneacetone)dipalladium(0) or is an organopalladium compound. The compound is a complex of palladium(0) with dibenzylideneacetone (dba). It is a dark-purple/brown solid, which is modestly soluble in organic solvents. Because the dba ligands are easily displaced, the complex is used as a homogeneous catalyst in organic synthesis.
Preparation and structure
First reported in 1970, it is prepared from dibenzylideneacetone and sodium tetrachloropalladate. Because it is commonly recrystallized from chloroform, the complex is often supplied as the adduct . The purity of samples can be variable.
In , the pair of Pd atoms are separated by 320 pm but are tied together by dba units. The Pd(0) centres are bound to the alkene parts of the dba ligands.
Applications
is used as a source of soluble Pd(0), in particular as a catalyst for various coupling reactions. Examples of reactions using this reagent are the Negishi coupling, Suzuki coupling, Carroll rearrangement, and Trost asymmetric allylic alkylation, as well as Buchwald–Hartwig amination.
Related Pd(0) complexes are and tetrakis(triphenylphosphine)palladium(0).
References
- ^ Jiro Tsuji and Ian J. S. Fairlamb "Tris(dibenzylideneacetone)dipalladium–Chloroform" E-EROS, 2008. doi:10.1002/047084289X.rt400.pub2
- Takahashi, Y.; Ito, Ts.; Sakai, S.; Ishii, Y. (1970). "A novel palladium(0) complex; bis(dibenzylideneacetone)palladium(0)". Journal of the Chemical Society D: Chemical Communications (17): 1065. doi:10.1039/C29700001065.
- Zalesskiy, S. S., Ananikov, V. P., "Pd2(dba)3 as a Precursor of Soluble Metal Complexes and Nanoparticles: Determination of Palladium Active Species for Catalysis and Synthesis", Organometallics 2012, 31, 2302. doi:10.1021/om201217r
- Pierpont, Cortlandt G.; Mazza, Margaret C. (1974). "Crystal and molecular structure of tris(dibenzylideneacetone)dipalladium(0)". Inorg. Chem. 13 (8): 1891. doi:10.1021/ic50138a020.
- Hartwig, J. F. (2010). Organotransition Metal Chemistry, from Bonding to Catalysis. New York: University Science Books. ISBN 978-1-891389-53-5.
- John R. Stille, F. Christopher Pigge, Christopher S. Regens, Ke Chen, Adrian Ortiz and Martin D. Eastgate "Bis(dibenzylideneacetone)palladium(0)" E-eros. 2013. doi:10.1002/047084289X.rb138.pub3
| Palladium compounds | |||
|---|---|---|---|
| Pd(0) |
| ||
| Pd(II) |
| ||
| Pd(II,IV) | |||
| Pd(IV) | |||
| Pd(VI) | |||