| |
| Names | |
|---|---|
| Preferred IUPAC name 3-Hydroxy-2-phenylpropanoic acid | |
| Other names 2-Phenylhydracrylic acid; Tropate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.201 |
| EC Number |
|
| KEGG | |
| MeSH | C011377 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C9H10O3 |
| Molar mass | 166.176 g·mol |
| Melting point | 116 °C (241 °F; 389 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Tropic acid is a chemical with IUPAC name 3-hydroxy-2-phenylpropanoic acid and condensed structural formula HOCH2CHPhCOOH. It is a laboratory reagent used in the chemical synthesis of atropine and hyoscyamine. Tropic acid is a chiral substance, existing as either a racemic mixture or as a single enantiomer.
Synthesis
Tropic acid can be prepared by the Ivanov reaction between phenylacetic acid and formaldehyde. In this method, the dianion of the acid is formed using a Grignard reagent, isopropyl magnesium chloride, and this reacts with the aldehyde to form the magnesium salt of the product, from which the pure acid is obtained after acidification with sulfuric acid.
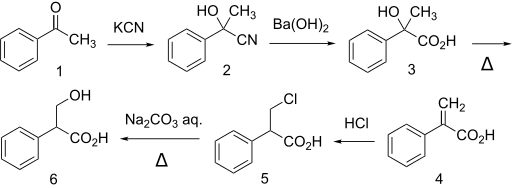
Many other methods have been used to make tropic acid, for example starting from acetophenone (1).
Uses
Tropic acid is used in the chemical synthesis of atropine and hyoscyamine.
References
- ^ Blicke, F. F.; Raffelson, Harold; Barna, Bohdan (1952). "The Preparation of Tropic Acid". Journal of the American Chemical Society. 74: 253. doi:10.1021/ja01121a504.
- Gadzikowska, M; Grynkiewicz, G (2002). "Tropane alkaloids in pharmaceutical and phytochemical analysis". Acta Poloniae Pharmaceutica. 59 (2): 149–60. PMID 12365608.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |