This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
Gas equation of state which accounts for non-ideal gas behavior

The van der Waals equation is a mathematical formula that describes the behavior of real gases. It is named after Dutch physicist Johannes Diderik van der Waals. It is an equation of state that relates the pressure, temperature, and molar volume in a fluid. However, it can be written in terms of other, equivalent, properties in place of the molar volume, for example specific volume, or number density. The equation modifies the ideal gas law in two ways. First its particles have a finite diameter, whereas the ideal gas consists of point particles with no extension. Second, its particles interact with one other, whereas the particles of an ideal gas move as though they were alone in the volume.
The surface calculated from the ideal gas equation of state is drawn in Fig. A. This universal (all ideal gases are represented by it) surface is normalized so that the black dot, with coordinates , appears at the location (1,1,1) of the 3 dimensional plot space. This device makes it easy to compare this surface with the one generated by the van der Waals equation in Fig. C. Figures A and C are drawn with the same scales and limits; they also present the two surfaces from the same viewpoint to make the comparison easier. Whereas the ideal gas surface is rather plain, the van der Waals surface has an interesting fold.

Figures B and C show different views of the surface calculated from the van der Waals equation. The fold seen on this surface is what enables the equation to predict the phenomenon of liquid--vapor phase change. This fold develops from a critical point defined by specific, critical, values of pressure, temperature, and molar volume. The surface is plotted using dimensionless variables that are formed by the ratio of each property to its respective critical value. This locates the critical point at the coordinates (1,1,1) of the space. When drawn using these dimensionless properties, this surface is, like that of the ideal gas, also universal. Moreover, it represents all real substances thttps://en.wikipedia.org/Van_der_Waals_equationo a remarkably high degree of accuracy. This principle of corresponding states, developed by van der Waals from his equation, has become one of the fundamental ideas in the thermodynamics of fluids.

The boundary of the fold on the surface is marked, on each side of the critical point, by the spinodal curve, identified in Fig. B, and seen in Figs. B and C. However, this curve does not define the location of the phase change. That place is given by the saturation curve, a curve that is not specified by the properties of the surface alone. The saturation curve is the locus of saturated liquid and vapor states which, being in equilibrium with each other, can coexist. The saturated liquid and vapor curves are identified in Fig. B. Together they comprise the saturation (or coexistence) curve seen in Figs. B and C. The inset in Fig. B shows the mixture states which are a combination of the saturated liquid and vapor states that exist at each end of the line, intersections of the mixture line and its isotherm. However, these mixture states are not part of the surface generated by the van der Waals equation; they are not solutions of the equation.
Although it is hard to imagine today when the quantum nature of physics is learned about early in life, it was only in 1909 that the scientific debate about the nature of matter, discrete or continuous, was finally settled. Indeed, at the time van der Waals created his equation, which he based on the idea that fluids are composed of discrete particles, few scientists believed that such particles really existed. They were regarded as purely metaphysical constructs that added nothing useful to the knowledge obtained from the results of experimental observations. However, the theoretical explanation of the critical point, which had been discovered a few years earlier, and later its qualitative and quantitative agreement with experiments cemented its acceptance in the scientific community. Ultimately these accomplishments won him the 1910 Nobel prize in physics. Today the equation is recognized as an important model of phase change processes. Van der Waals also adapted his equation so that it applied to a binary mixture of fluids. He, and others, then used the modified equation to discover a host of important facts about the phase equilibria of such fluids. This application, expanded to treat multi-component mixtures, has extended the predictive ability of the equation to fluids of industrial and commercial importance. In this arena it has spawned many similar equations in a continuing attempt by engineers to improve their ability to understand and manage these fluids; it remains relevant to the present.
Behavior of the equation
One way to write the van der Waals equation is:
where is pressure, is temperature, and is molar volume. In addition is the Avogadro constant, is the volume, and is the number of molecules (the ratio is a physical quantity with base unit mole (symbol mol) in the SI). Finally is the universal gas constant, is the Boltzmann constant, and and are experimentally determinable, substance-specific constants.
As noted previously, when van der Waals created his equation the idea that fluids were composed of rapidly moving particles was believed by very few scientists. Moreover, those who thought so had no knowledge of the atomic/molecular structure. The simplest conception, and the easiest to model mathematically was a hard sphere and that is what van der Waals used. In that case two particles of diameter, , would come into contact when their centers were a distance apart, hence the center of the one was excluded from a spherical volume equal to about the other. That is 8 times , the volume of each particle of radius , but there are 2 particles which gives 4 times the volume per particle. The total excluded volume is then , 4 times the volume of all the particles. Van der Waals and his contemporaries used an alternative, but equivalent, analysis based on the mean free path between molecular collisions that gave this result. From the fact that the volume fraction of particles, must be positive, van der Waals noted that as becomes larger the factor 4 must decrease (for spheres there is a known minimum ), but he was never able to determine the nature of the decrease. The constant in the equation above has dimension molar volume, . The constant expresses the strength of the hypothesized interparticle attraction. Van der Waals only had as a model Newton's law of gravitation, in which two particles attracted in proportion to the product of their masses. Thus he argued that in his case the attractive pressure was proportional to the square of the density. The proportionality constant, , when written in the form used above, has dimension pressure times molar volume squared, which is also molar energy times molar volume.

The intermolecular force was later conveniently described by the negative derivative of a pair potential function. For spherically symmetric particles this is most simply a function of separation distance with a single characteristic length, , and a minimum energy, (with ). Two of the many such functions that have been suggested are shown in the accompanying plot.
A modern theory based on statistical mechanics produces the same result for obtained by van der Waals and his contemporaries. This result is valid for any pair potential for which the increase in is sufficiently rapid. This includes the hard sphere model for which the increase is infinitely rapid and the result is exact. Indeed the Sutherland potential most accurately models van der Waals' conception of a molecule. It also includes potentials that do not represent hard sphere force interactions provided that the increase in is fast enough, but then it is approximate; increasingly better the faster the increase. In that case is only an "effective diameter" of the molecule. This theory also produces where is a number that depends on the shape of the potential function, . However, this result is only valid when the potential is weak, namely, when the minimum potential energy is very much smaller than the thermal energy, .
In his book (see references and ) Boltzmann wrote equations using (specific volume) in place of (molar volume) used here, Gibbs did as well, so do most engineers. Also the property, the reciprocal of number density, is used by physicists, but there is no essential difference between equations written with any of these properties. Equations of state written using molar volume contain , those using specific volume contain (the substance specific is the molar mass with the mass of a single particle), and those written with number density contain .
Once and are experimentally determined for a given substance, the van der Waals equation can be used to predict the boiling point at any given pressure, the critical point (defined by pressure and temperature values, , such that the substance cannot be liquefied either when no matter how low the temperature, or when no matter how high the pressure; uniquely define ), and other attributes. These predictions are accurate for only a few substances. For most simple fluids they are only a valuable approximation. The equation also explains why superheated liquids can exist above their boiling point and subcooled vapors can exist below their condensation point.

The graph on the right follows from the intersection of the surface shown in Figs. B and C and 4 constant pressure planes. Each intersection produces a curve in the plane corresponding to the value of the pressure chosen.
On the red isobar (another name for a constant pressure curve), , the slope is positive over the entire range, (although the plot only shows a finite quadrant). This describes a fluid as homogeneous for all , and is characteristic of all supercritical isobars
The green isobar, , has a region of negative slope. This region consists of states that are unstable and therefore never observed (for this reason this region is shown dotted gray). The green curve thus consists of two disconnected branches; a vapor on the right, and a denser liquid on the left. For this pressure, at a temperature, , specified by mechanical, thermal, and material equilibrium, and shown as green circles on the curve, the boiling (saturated) liquid, , (the left circle) and condensing (saturated) vapor, , (the right circle) coexist. Due to gravity the denser liquid appears below the vapor, and a meniscus is seen between them. This heterogeneous combination of coexisting liquid and vapor is the phase change. Heating the fluid in this state increases the fraction of vapor in the mixture; its , an average of and weighted by this fraction, increases while remains the same. This is shown as the dotted gray line, because it does not represent a solution of the equation; however, it does describe the observed behavior. The points above , superheated liquid, and those below it, subcooled vapor, are metastable; a sufficiently strong disturbance causes them to transform to the stable alternative. Consequently they are shown dashed.
All this describes a fluid as a stable vapor for , a stable liquid for , and a mixture of liquid and vapor at , that also supports metastable states of subcooled vapor and superheated liquid. It is characteristic of all subcritical isobars , where is a function of .
The orange isobar is the critical one on which the minimum and maximum are equal. The critical point lies on this isobar.
The black isobar is the limit of positive pressures, although drawn solid none of its points represent stable solutions, they are either metastable (positive or zero slope) or unstable (negative slope). Interestingly, states of negative pressure (tension) exist. They lie below the black isobar, and although they are not not drawn in this figure, they form those parts of the surfaces seen in Figs. B and C that lie below the zero pressure plane. In this, , plane they have a parabola like shape and like the zero pressure isobar their states are all either metastable (positive or zero slope) or unstable (negative slope).
Relationship to the ideal gas law
The ideal gas law follows from the van der Waals equation whenever is sufficiently large (or correspondingly whenever the molar density, , is sufficiently small), Specifically
- when , then is numerically indistinguishable from ,
- and when , then is numerically indistinguishable from .
Putting these two approximations into the van der Waals equation when is large enough that both inequalities are satisfied reduces it to
which is the ideal gas law. This is not surprising since the van der Waals equation was constructed from the ideal gas equation in order to obtain an equation valid beyond the limit of ideal gas behavior.
What is truly remarkable is the extent to which van der Waals succeeded. Indeed, Epstein in his classic thermodynamics textbook began his discussion of the van der Waals equation by writing, "In spite of its simplicity, it comprehends both the gaseous and the liquid state and brings out, in a most remarkable way, all the phenomena pertaining to the continuity of these two states". Also in Volume 5 of his Lectures on Theoretical Physics Sommerfeld, in addition to noting that "Boltzmann described van der Waals as the Newton of real gases", also wrote "It is very remarkable that the theory due to van der Waals is in a position to predict, at least qualitatively, the unstable states" that are associated with the phase change process.
Utility of the equation
The equation has been, and remains very useful because:
- its specific heat at constant volume, , can be shown to be a function of only, and its thermodynamic properties, internal energy , entropy , as well as the specific heat at constant pressure have simple analytic expressions [this is also true of enthalpy , Helmholtz free energy , and Gibbs free energy ]
- Its coefficient of thermal expansion, has a simple analytic expression [this is also true of its isothermal compressibility, ]
- it explains the existence of the critical point and the liquid–vapor phase transition including the observed metastable states
- it establishes the law of corresponding states
- its Joule–Thomson coefficient and associated inversion curve, which were instrumental in the development of the commercial liquefaction of gases, have simple analytic expressions.
In addition its vapor pressure curve (also called the coexistence, or saturation, curve) has a simple analytic solution. It depicts the liquid metals, Mercury and Cesium, quantitatively, and describes most real fluids qualitatively. Consequently it can be regarded as one member of a family of equations of state, that depend on a molecular parameter such as the critical compressibility factor, , or the Pitzer (acentric) factor, , where is a dimensionless saturation pressure, and log is the logarithm base 10. Consequently, the equation plays an important role in the modern theory of phase transitions.
All this makes it a worthwhile pedagogical tool for physics, chemistry, and engineering lecturers, in addition to being a useful mathematical model which can aid student understanding.
History
In 1857 Rudolf Clausius published The Nature of the Motion which We Call Heat. In it he derived the relation for the pressure, , in a gas, composed of particles in motion, with number density , mass , and mean square speed . He then noted that using the classical laws of Boyle and Charles one could write with a constant of proportionality. Hence temperature was proportional to the average kinetic energy of the particles. This article inspired further work based on the twin ideas that substances are composed of indivisible particles, and that heat is a consequence of the particle motion; movement that evolves in accordance with Newton's laws. The work, known as the kinetic theory of gases, was done principally by Clausius, James Clerk Maxwell, and Ludwig Boltzmann. At about the same time J. Willard Gibbs also contributed, and advanced it by converting it into statistical mechanics.

This environment influenced Johannes Diderik van der Waals. After initially pursuing a teaching credential, he was accepted for doctoral studies at the University of Leiden under Pieter Rijke. This led, in 1873, to a dissertation that provided a simple, particle based, equation that described the gas–liquid change of state, the origin of a critical temperature, and the concept of corresponding states. The equation is based on two premises, first that fluids are composed of particles with non-zero volumes, and second that at a large enough distance each particle exerts an attractive force on all other particles in its vicinity. These forces were called by Boltzmann van der Waals cohesive forces.
In 1869 Irish professor of chemistry Thomas Andrews at Queen's University Belfast in a paper entitled On the Continuity of the Gaseous and Liquid States of Matter, displayed an experimentally obtained set of isotherms of carbonic acid, HCO, that showed at low temperatures a jump in density at a certain pressure, while at higher temperatures there was no abrupt change; the figure can be seen here. Andrews called the isotherm at which the jump just disappeared the critical point. Given the similarity of the titles of this paper and van der Waals subsequent thesis one might think that van der Waals set out to develop a theoretical explanation of Andrews' experiments; however, this is not what happened. Van der Waals began work by trying to determine a mollecular attraction that appeared in Laplace's theory of capillarity, and only after establishing his equation he tested it using Andrews results.
By 1877 sprays of both liquid oxygen and liquid nitrogen had been produced, and a new field of research, low temperature physics, had been opened. The van der Waals equation played a part in all this especially with respect to the liquefaction of hydrogen and helium which was finally achieved in 1908. From measurements of and in two states with the same density, the van der Waals equation produces the values,
Thus from two such measurements of pressure and temperature one could determine and , and from these values calculate the expected critical pressure, temperature, and molar volume. Goodstein summarized this contribution of the van der Waals equation as follows:
All this labor required considerable faith in the belief that gas–liquid systems were all basically the same, even if no one had ever seen the liquid phase. This faith arose out of the repeated success of the van der Waals theory, which is essentially a universal equation of state, independent of the details of any particular substance once it has been properly scaled. ... As a result, not only was it possible to believe that hydrogen could be liquefied. but it was even possible to predict the necessary temperature and pressure.
Van der Waals was awarded the Nobel Prize in 1910, in recognition of the contribution of his formulation of this "equation of state for gases and liquids".
As noted previously, modern day studies of first order phase changes make use of the van der Waals equation together with the Gibbs criterion, equal chemical potential of each phase, as a model of the phenomenon. This model has an analytic coexistence (saturation) curve expressed parametrically, (the parameter is related to the entropy difference between the two phases), that was first obtained by Plank, was known to Gibbs and others, and was later derived in a beautifully simple and elegant manner by Lekner. A summary of Lekner's solution is presented in a subsequent section, and a more complete discussion in the Maxwell construction.
Critical point and corresponding states
Figure 1 shows four isotherms of the van der Waals equation (abbreviated as vdW) on a pressure, molar volume plane. The essential character of these curves is that:
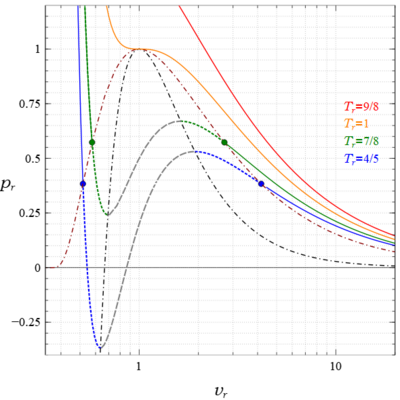
- at some critical temperature, the slope is negative, , everywhere except at a single point, the critical point, , where both the slope and curvature are zero,
- at higher temperatures the slope of the isotherms is everywhere negative (values of for which the equation has 1 real root for );
- at lower temperatures there are two points on each isotherm where the slope is zero (values of , for which the equation has 3 real roots for )
Evaluating the two partial derivatives in 1) using the vdW equation and equating them to zero produces, , and using these in the equation gives .
This calculation can also be done algebraically by noting that the vdW equation can be written as a cubic in , which at the critical point is,
Moreover, at the critical point all three roots coalesce so it can also be written as
Then dividing the first by , and noting that these two cubic equations are the same when all their coefficients are equal gives three equations, , whose solution produces the previous results for .
Using these critical values to define reduced properties renders the equation in the dimensionless form used to construct Fig. 1
This dimensionless form is a similarity relation; it indicates that all vdW fluids at the same will plot on the same curve. It expresses the law of corresponding states which Boltzmann described as follows:
All the constants characterizing the gas have dropped out of this equation. If one bases measurements on the van der Waals units , then he obtains the same equation of state for all gases. ... Only the values of the critical volume, pressure, and temperature depend on the nature of the particular substance; the numbers that express the actual volume, pressure, and temperature as multiples of the critical values satisfy the same equation for all substances. In other words, the same equation relates the reduced volume, reduced pressure, and reduced temperature for all substances.
Obviously such a broad general relation is unlikely to be correct; nevertheless, the fact that one can obtain from it an essentially correct description of actual phenomena is very remarkable.
This "law" is just a special case of dimensional analysis in which an equation containing 6 dimensional quantities, , and 3 independent dimensions, , , (independent means that "none of the dimensions of these quantities can be represented as a product of powers of the dimensions of the remaining quantities", and =), must be expressible in terms of 6 − 3 = 3 dimensionless groups. Here is a characteristic molar volume, a characteristic pressure, and a characteristic temperature, and the 3 dimensionless groups are . According to dimensional analysis the equation must then have the form , a general similarity relation. In his discussion of the vdW equation Sommerfeld also mentioned this point. The reduced properties defined previously are , , and . Recent research has suggested that there is a family of equations of state that depend on an additional dimensionless group, and this provides a more exact correlation of properties. Nevertheless, as Boltzmann observed, the van der Waals equation provides an essentially correct description.
The vdW equation produces , while for most real fluids . Thus most real fluids do not satisfy this condition, and consequently their behavior is only described qualitatively by the vdW equation. However, the vdW equation of state is a member of a family of state equations based on the Pitzer (acentric) factor, , and the liquid metals, Mercury and Cesium, are well approximated by it.
Thermodynamic properties
The properties molar internal energy, , and entropy, , defined by the first and second laws of thermodynamics, hence all thermodynamic properties of a simple compressible substance, can be specified, up to a constant of integration, by two measurable functions, a mechanical equation of state, , and a constant volume specific heat, .
Internal energy and specific heat at constant volume
The internal energy is given by the energetic equation of state,
where is an arbitrary constant of integration.
Now in order for to be an exact differential, namely that be continuous with continuous partial derivatives, its second mixed partial derivatives must also be equal, . Then with this condition can be written simply as . Differentiating for the vdW equation gives , so . Consequently for a vdW fluid exactly as it is for an ideal gas. To keep things simple it is regarded as a constant here, with a number. Then both integrals can be easily evaluated and the result is
This is the energetic equation of state for a perfect vdW fluid. By making a dimensional analysis (what might be called extending the principle of corresponding states to other thermodynamic properties) it can be written simply in reduced form as,
where and is a dimensionless constant.
Enthalpy
The enthalpy is , and the product is just . Then
is simply
This is the enthalpic equation of state for a perfect vdW fluid, or in reduced form,
Entropy
The entropy is given by the entropic equation of state:
Using as before, and integrating the second term using we obtain simply
This is the entropic equation of state for a perfect vdW fluid, or in reduced form,
Helmholtz free energy
The Helmholtz free energy is so combining the previous results
This is the Helmholtz free energy for a perfect vdw fluid, or in reduced form
Gibbs free energy
The Gibbs free energy is so combining the previous results gives
This is the Gibbs free energy for a perfect vdW fluid, or in reduced form
Thermodynamic derivatives: α, κT and cp
The two first partial derivatives of the vdW equation are
Here , the isothermal compressibility, is a measure of the relative increase of volume from an increase of pressure, at constant temperature, while , the coefficient of thermal expansion, is a measure of the relative increase of volume from an increase of temperature, at constant pressure. Therefore,
In the limit while . Since the vdW equation in this limit becomes , finally . Both of these are the ideal gas values, which is consistent because, as noted earlier, the vdW fluid behaves like an ideal gas in this limit.
The specific heat at constant pressure, is defined as the partial derivative . However, it is not independent of , they are related by the Mayer equation, . Then the two partials of the vdW equation can be used to express as,
Here in the limit , , which is also the ideal gas result as expected; however the limit gives the same result, which does not agree with experiments on liquids.
In this liquid limit we also find , namely that the vdW liquid is incompressible. Moreover, since , it is also mechanically incompressible, that is faster than .
Finally , and are all infinite on the curve . This curve, called the spinodal curve, is defined by , and is discussed at length in the next section.
Stability
According to the extremum principle of thermodynamics and , namely that at equilibrium the entropy is a maximum. This leads to a requirement that . This mathematical criterion expresses a physical condition which Epstein described as follows:

"It is obvious that this middle part, dotted in our curves , can have no physical reality. In fact, let us imagine the fluid in a state corresponding to this part of the curve contained in a heat conducting vertical cylinder whose top is formed by a piston. The piston can slide up and down in the cylinder, and we put on it a load exactly balancing the pressure of the gas. If we take a little weight off the piston, there will no longer be equilibrium and it will begin to move upward. However, as it moves the volume of the gas increases and with it its pressure. The resultant force on the piston gets larger, retaining its upward direction. The piston will, therefore, continue to move and the gas to expand until it reaches the state represented by the maximum of the isotherm. Vice versa, if we add ever so little to the load of the balanced piston, the gas will collapse to the state corresponding to the minimum of the isotherm"
While on an isotherm this requirement is satisfied everywhere so all states are gas, those states on an isotherm, which lie between the local minimum, , and local maximum, , for which (shown dashed gray in Fig. 1), are unstable and thus not observed. This is the genesis of the phase change; there is a range , for which no observable states exist. The states for are liquid, and for are vapor; the denser liquid lies below the vapor due to gravity. The transition points, states with zero slope, are called spinodal points. Their locus is the spinodal curve that separates the regions of the plane for which liquid, vapor, and gas exist from a region where no observable homogeneous states exist. This spinodal curve is obtained here from the vdW equation by differentiation (or equivalently from ) as
A projection of this space curve is plotted in Fig. 1 as the black dash dot curve. It passes through the critical point which is also a spinodal point.
Saturation
Although the gap in delimited by the two spinodal points on an isotherm (e.g. shown in Fig. 1) is the origin of the phase change, the spinodal points do not represent its full extent, because both states, saturated liquid and saturated vapor coexist in equlilbrium; they both must have the same pressure as well as the same temperature. Thus the phase change is characterized, at temperature , by a pressure that lies between that of the minimum and maximum spinodal points, and with molar volumes of liquid, and vapor . Then from the vdW equation applied to these saturated liquid and vapor states
These two vdW equations contain 4 variables, , so another equation is required in order to specify the values of 3 of these variables uniquely in terms of a fourth. Such an equation is provided here by the equality of the Gibbs free energy in the saturated liquid and vapor states, . This condition of material equilibrium can be obtained from a simple physical argument as follows: the energy required to vaporize a mole is from the second law at constant temperature , and from the first law at constant pressure . Equating these two, rearranging, and recalling that produces the result.
The Gibbs free energy is one of the 4 thermodynamic potentials whose partial derivatives produce all other thermodynamics state properties; its differential is . Integrating this over an isotherm from to , noting that the pressure is the same at each endpoint, and setting the result to zero yields
Here because is a multivalued function, the integral must be divided into 3 parts corresponding to the 3 real roots of the vdW equation in the form, (this can be visualized most easily by imagining Fig. 1 rotated ); the result is a special case of material equilibrium. The last equality, which follows from integrating , is the Maxwell equal area rule which requires that the upper area between the vdW curve and the horizontal through be equal to the lower one. This form means that the thermodynamic restriction that fixes is specified by the equation of state itself, . Using the equation for the Gibbs free energy obtained previously for the vdW equation applied to the saturated vapor state and subtracting the result applied to the saturated liquid state produces,
This is a third equation that along with the two vdW equations above can be solved numerically. This has been done given a value for either or , and tabular results presented; however, the equations also admit an analytic parametric solution obtained most simply and elegantly, by Lekner. Details of this solution may be found in the Maxwell Construction; the results are
where

and the parameter is given physically by . The values of all other property discontinuities across the saturation curve also follow from this solution. These functions define the coexistence curve which is the locus of the saturated liquid and saturated vapor states of the vdW fluid. The curve is plotted in Fig. 1 and Fig. 2, two projections of the state surface. These curves and the numerical results referenced earlier agree exactly, as they must.
Referring back to Fig. 1 the isotherms for are discontinuous. Considering as an example, it consists of the two separate green segments. The solid segment above the green circle on the left, and below the one on the right correspond to stable states, the dots represent the saturated liquid and vapor states that comprise the phase change, and the two green dotted segments below and above the dots are metastable states, superheated liquid and subcooled vapor, that are created in the process of phase transition, have a short lifetime, then devolve into their lower energy stable alternative.
In his treatise of 1898 in which he described the van der Waals equation in great detail Boltzmann discussed these states in a section titled "Undercooling, Delayed evaporation"; they are now denoted subcooled vapor, and superheated liquid. Moreover, it has now become clear that these metastable states occur regularly in the phase transition process. In particular processes that involve very high heat fluxes create large numbers of these states, and transition to their stable alternative with a corresponding release of energy can be dangerous. Consequently there is a pressing need to study their thermal properties.
In the same section Boltzmann also addressed and explained the negative pressures which some liquid metastable states exhibit (for example of Fig. 1). He concluded that such liquid states of tensile stresses were real, as did Tien and Lienhard many years later who wrote "The van der Waals equation predicts that at low temperatures liquids sustain enormous tension...In recent years measurements have been made that reveal this to be entirely correct."
Even though the phase change produces a mathematical discontinuity in the homogeneous fluid properties, for example , there is no physical discontinuity. As the liquid begins to vaporize the fluid becomes a heterogeneous mixture of liquid and vapor whose molar volume varies continuously from to according to the equation of state

where is the mole fraction of the vapor. This equation is called the lever rule and applies to other properties as well. The states it represents form a horizontal line connecting the same colored dots on an isotherm, but not shown in Fig. 1 as noted already since it is a distinct equation of state for the heterogeneous combination of liquid and vapor components.
Extended corresponding states
The idea of corresponding states originated when van der Waals cast his equation in the dimensionless form, . However, as Boltzmann noted, such a simple representation could not correctly describe all substances. Indeed, the saturation analysis of this form produces , namely all substances have the same dimensionless coexistence curve. In order to avoid this paradox an extended principle of corresponding states has been suggested in which where is a substance dependent dimensionless parameter related to the only physical feature associated with an individual substance, its critical point.

The most obvious candidate for is the critical compressibility factor , but because is difficult to measure accurately, the acentric factor developed by Kenneth Pitzer, , is more useful. The saturation pressure in this situation is represented by a one parameter family of curves, . Several investigators have produced correlations of saturation data for a number of substances, the best is that of Dong and Lienhard,
which has an rms error of over the range
Figure 3 is a plot of vs . for various values of as given by this equation. The ordinate is logarithmic in order to show the behavior at pressures far below the critical where differences among the various substances (indicated by varying values of ) are more pronounced.
Figure 4 is another plot of the same equation showing as a function of for various values of . It includes data from 51 substances, including the vdW fluid, over the range . This plot shows clearly that the vdW fluid () is a member of the class of real fluids; indeed it quantitatively describes the behavior of the liquid metals cesium () and mercury () whose values of are close to the vdW value. However, it describes the behavior of other fluids only qualitatively, because specific numerical values are modified by differing values of their Pitzer factor, .
Joule–Thomson coefficient
The Joule–Thomson coefficient, , is of practical importance because the two end states of a throttling process () lie on a constant enthalpy curve. Although ideal gases, for which , do not change temperature in such a process, real gases do, and it is important in applications to know whether they heat up or cool down.
This coefficient can be found in terms of the previously described derivatives as,
so when is positive the gas temperature decreases when it passes through a throttle, and if it is negative the temperature increases. Therefore the condition defines a curve that separates the region of the plane where from the region where it is less than zero. This curve is called the inversion curve, and its equation is . Using the expression for derived previously for the van der Waals equation this is
Note that for there will be cooling for or in terms of the critical temperature . As Sommerfeld noted, "This is the case with air and with most other gases. Air can be cooled at will by repeated expansion and can finally be liquified."

In terms of the equation has a simple positive solution which, for produces, . Using this to eliminate from the vdW equation then gives the inversion curve as
where, for simplicity, have been replaced by .
The maximum of this, quadratic, curve occurs, with , for
which gives , or , and the corresponding . The zeros of the curve , are, making use of the quadratic formula, , or and ( and ). In terms of the dimensionless variables, the zeros are at and , while the maximum is , and occurs at . A plot of the curve is shown in green in Fig. 5. Sommerfeld also displays this plot, together with a curve drawn using experimental data from H2. The two curves agree qualitatively, but not quantitatively. For example the maximum on these two curves differ by about 40% in both magnitude and location.
Figure 5 shows an overlap between the saturation curve and the inversion curve plotted there. This region is shown enlarged in the right hand graph of the figure. Thus a van der Waals gas can be liquified by passing it through a throttle under the proper conditions; real gases are liquified in this way.
Compressibility factor

Real gases are characterized by their difference from ideal by writing . Here , called the compressibility factor, is expressed either as or . In either case
takes the ideal gas value. In the second case , so for a van der Waals fluid the compressibility factor is simply , or in terms of reduced variables
where . At the critical point, , .
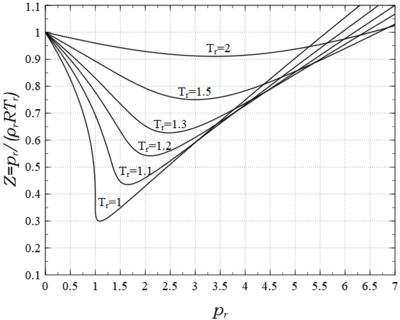
In the limit , ; the fluid behaves like an ideal gas, a point noted several times earlier. The derivative is never negative when , namely when (). Alternatively when the initial slope is negative, it becomes zero at , and is positive for larger (see Fig. 6). In this case the value of passes through when . Here is called the Boyle temperature. It varies between , and denotes a point in space where the equation of state reduces to the ideal gas law. However the fluid does not behave like an ideal gas there, because neither its derivatives reduce to their ideal gas values, other than where the actual ideal gas region.
Figure 6 shows a plot of various isotherms of vs . Also shown are the spinodal and coexistence curves described previously. The subcritical isotherm consists of stable, metastable, and unstable segments, and are identified the same as they were in Fig. 1. Also included are the zero initial slope isotherm and the one corresponding to infinite temperature.
By plotting vs using as a parameter, one obtains the generalized compressibility chart for a vdW gas, which is shown in Fig. 7. Like all other vdW properties, this is not quantitatively correct for most gases but it has the correct qualitative features as can be seen by comparison with this figure which was produced from data using real gases. The two graphs are similar, including the caustic generated by the crossing isotherms; they are qualitatively very much alike.
Virial expansion
Statistical mechanics suggests that can be expressed by a power series called a virial expansion,
The functions are the virial coefficients; the th term represents a particle interaction.
Expanding the term in the compressibility factor of the vdW equation in its infinite series, convergent for , produces
The corresponding expression for when is
These are the virial expansions, one dimensional and one dimensionless, for the van der Waals fluid. The second virial coefficient is the slope of at . Notice that it can be positive or negative depending on whether or not , which agrees with the result found previously by differentiation.
For molecules that are non attracting hard spheres, , the vdW virial expansion becomes simply
which illustrates the effect of the excluded volume alone. It was recognized early on that this was in error beginning with the term . Boltzmann calculated its correct value as , and used the result to propose an enhanced version of the vdW equation
On expanding , this produced the correct coefficients thru and also gave infinite pressure at , which is approximately the close packing distance for hard spheres. This was one of the first of many equations of state proposed over the years that attempted to make quantitative improvements to the remarkably accurate explanations of real gas behavior produced by the vdW equation.
Mixtures
In 1890 van der Waals published an article that initiated the study of fluid mixtures. It was subsequently included as Part III of a later published version of his thesis. His essential idea was that in a binary mixture of vdw fluids described by the equations
the mixture is also a vdW fluid given by
where
Here , and , with (so that ) are the mole fractions of the two fluid substances. Adding the equations for the two fluids shows that , although for sufficiently large with equality holding in the ideal gas limit. The quadratic forms for and are a consequence of the forces between molecules. This was first shown by Lorentz, and was credited to him by van der Waals. The quantities and in these expressions characterize collisions between two molecules of the same fluid component while and represent collisions between one molecule of each of the two different component fluids. This idea of van der Waals was later called a one fluid model of mixture behavior.
Assuming that is the arithmetic mean of and , , substituting into the quadratic form, and noting that produces
Van der Waals wrote this relation, but did not make use of it initially. However, it has been used frequently in subsequent studies, and its use is said to produce good agreement with experimental results at high pressure.
Common Tangent Construction
In this article van der Waals used the Helmholtz Potential Minimum Principle to establish the conditions of stability. This principle states that in a system in diathermal contact with a heat reservoir , and , namely at equilibrium the Helmholtz potential is a minimimum. Since, like , the molar Helmholtz function is also a potential function whose differential is
this minimum principle leads to the stability condition . This condition means that the function, , is convex at all stable states of the system. Moreover, for those states the previous stability condition for the pressure is necessarily satisfied as well.
For a single substance the definition of the molar Gibbs free energy can be written in the form . Thus when and are constant along with temperature the function represents a straight line with slope , and intercept . Since the curve, , has positive curvature everywhere when , the curve and the straight line will have a single tangent. However, for a subcritical is not everwhere convex. With and a suitable value of the line will be tangent to at the molar volume of each coexisting phase, saturated liquid, , and saturated vapor, ; there will be a double tangent. Furthermore, each of these points is characterized by the same value of as well as the same values of and These are the same three specifications for coexistence that were used previously.

As depicted in Fig. 8, the region on the green curve for ( is designated by the left green circle) is the liquid. As increases past the curvature of (proportional to ) continually decreases. The point characterized by , is a spinodal point, and between these two points is the metastable superheated liquid. For further increases in the curvature decreases to a minimum then increases to another spinodal point; between these two spinodal points is the unstable region in which the fluid cannot exist in a homogeneous equilibrium state. With a further increase in the curvature increases to a maximum at , where the slope is ; the region between this point and the second spinodal point is the metastable subcooled vapor. Finally, the region is the vapor. In this region the curvature continually decreases until it is zero at infinitely large . The double tangent line is rendered solid between its saturated liquid and vapor values to indicate that states on it are stable, as opposed to the metastable and unstable states, above it (with larger Helmholtz free energy), but black, not green, to indicate that these states are heterogeneous, not homogeneous solutions of the vdW equation. The combined green black curve in Fig. 8 is the convex envelope of , which is defined as the largest convex curve that is less than or equal to the function.
For a vdW fluid the molar Helmholtz potential is
where . Its derivative is
which is the vdW equation, as it must be. A plot of this function , whose slope at each point is specified by the vdW equation, for the subcritical isotherm is shown in Fig. 8 along with the line tangent to it at its two coexisting saturation points. The data illustrated in Fig. 8 is exactly the same as that shown in Fig.1 for this isotherm. This double tangent construction thus provides a simple graphical aternative to the Maxwell construction to establish the saturated liquid and vapor points on an isotherm.
Van der Waals used the Helmholtz function because its properties could be easily extended to the binary fluid situation. In a binary mixture of vdW fluids the Helmholtz potential is a function of 2 variables, , where is a composition variable, for example so . In this case there are three stability conditions
and the Helmholtz potential is a surface (of physical interest in the region ). The first two stability conditions show that the curvature in each of the directions and are both non negative for stable states while the third condition indicates that stable states correspond to elliptic points on this surface. Moreover its limit,
specifies the spinodal curves on the surface.
For a binary mixture the Euler equation, can be written in the form
Here are the molar chemical potentials of each substance, . For , and , all constant this is the equation of a plane with slopes in the direction, in the direction, and intercept . As in the case of a single substance, here the plane and the surface can have a double tangent and the locus of the coexisting phase points forms a curve on each surface. The coexistence conditions are that the two phases have the same , , , and ; the last two are equivalent to having the same and individually, which are just the Gibbs conditions for material equilibrium in this situation. The two methods of producing the coexistence surface are equivalent
Although this case is similar to the previous one of a single component, here the geometry can be much more complex. The surface can develop a wave (called a plait or fold in the literature) in the direction as well as the one in the direction. Therefore, there can be two liquid phases that can be either miscible, or wholly or partially immiscible, as well as a vapor phase. Despite a great deal of both theoretical and experimental work on this problem by van der Waals and his successors, work which produced much useful knowledge about the various types of phase equilibria that are possible in fluid mixtures, complete solutions to the problem were only obtained after 1967, when the availability of modern computers made calculations of mathematical problems of this complexity feasible for the first time. The results obtained were, in Rowlinson's words,
a spectacular vindication of the essential physical correctness of the ideas behind the van der Waals equation, for almost every kind of critical behavior found in practice can be reproduced by the calculations, and the range of parameters that correlate with the different kinds of behavior are intelligible in terms of the expected effects of size and energy.
Mixing Rules
In order to obtain these numerical results the values of the constants of the individual component fluids must be known. In addition, the effect of collisions between molecules of the different components, given by and , must also be specified. In he absence of experimental data, or computer modeling results to estimate their value the empirical combining rules,
the geometric and algebraic means respectively can be used. These relations correspond to the empirical combining rules for the intermolecular force constants,
the first of which follows from a simple interpretation of the dispersion forces in terms of polarizabilities of the individual molecules while the second is exact for rigid molecules. Then, generalizing for fluid components, and using these empirical combinig laws, the quadradic mixing rules for the material constants are:
Using similar expressions in the vdW equation is apparently helpful for divers. They are also important for physical scientists, and engineers in their study and management of the various phase equilibria and critical behavior observed in fluid mixtures. However more sophisticated mixing rules have often been found to be necessary, in order to obtain satisfactory agreement with reality over the wide variety of mixtures encountered in practice.
Another method of specifying the vdW constants pioneered by W.B. Kay, and known as Kay's rule. specifies the effective critical temperature and pressure of the fluid mixture by
In terms of these quantities the vdW mixture constants are then,
and Kay used these specifications of the mixture critical constants as the basis for calculations of the thermodynamic properties of mixtures.
Kay's idea was adopted by T. W. Leland, who applied it to the molecular parameters, , which are related to through by and . Using these together with the quadratic mixing rules for produces
which is the van der Waals approximation expressed in terms of the intermolecular constants. This approximation, when compared with computer simulations for mixtures, are in good agreement over the range , namely for molecules of not too different diameters. In fact Rowlinson said of this approximation, "It was, and indeed still is, hard to improve on the original van der Waals recipe when expressed in form".
Mathematical and Empirical Validity
Since van der Waals presented his thesis, "any derivations, pseudo-derivations, and plausibility arguments have been given" for it. However, no mathematically rigorous derivation of the equation over its entire range of molar volume that begins from a statistical mechanical principle exists. Indeed, such a proof is not possible, even for hard spheres. Goodstein put it this way, "Obviously the value of the van der Waals equation rests principally on its empirical behavior rather than its theoretical foundation."
Nevertheless a review of the work that has been done is useful in order to better understand where and when the equation is valid mathematically, and where and why it fails.
Review
The classical canonical partition function, , of statistical mechanics for a three dimensional particle macroscopic system is, Here , is the DeBroglie wavelength (alternatively is the quantum concentration), is the particle configuration integral, and is the intermolecular potential energy, which is a function of the particle position vectors . Lastly is the volume element of , which is a dimensional space.
The connection of with thermodynamics is made through the Helmholtz free energy, from which all other properties can be found; in particular . For point particles that have no force interactions, , all integrals of can be evaluated producing . In the thermodynamic limit, with finite, the Helmholtz free energy per particle (or per mole, or per unit mass) is finite, for example per mole it is . The thermodynamic state equations in this case are those of a monatomic ideal gas, specifically
Early derivations of the vdW equation were criticized mainly on two grounds; 1) a rigorous derivation from the partition function should produce an equation that does not include unstable states for which, ; 2) the constant in the vdw equation (here is the volume of a single molecule) gives the maximum possible number of molecules as , or a close packing density of 1/4=0.25, whereas the known close packing density of spheres is . Thus a single value of cannot describe both gas and liquid states.
The second criticism is an indication that the vdW equation cannot be valid over the entire range of molar volume. Van der Waals was well aware of this problem; he devoted about 30% of his Nobel lecture to it, and also said that it is
... the weak point in the study of the equation of state. I still wonder whether there is a better way. In fact this question continually obsesses me, I can never free myself from it, it is with me even in my dreams.
In 1949 the first criticism was proved by van Hove when he showed that in the thermodynamic limit hard spheres with finite range attractive forces have a finite Helmholtz free energy per particle. Furthermore this free energy is a continuously decreasing function of the volume per particle, (see Fig. 8 where are molar quantities). In addition its derivative exists and defines the pressure, which is a non increasing function of the volume per particle. Since the vdW equation has states for which the pressure increases with increasing volume per particle, this proof means it cannot be derived from the partition function, without an additional constraint that precludes those states.
In 1891 Korteweg showed using kinetic theory ideas, that a system of hard rods of length , constrained to move along a straight line of length , and exerting only direct contact forces on one another satisfy a vdW equation with ; Rayleigh also knew this. Later Tonks, by evaluating the configuration integral, showed that the force exerted on a wall by this system is given by, This can be put in a more recognizable, molar, form by dividing by the rod cross sectional area , and defining . This produces ; clearly there is no condensation, for all . This simple result is obtained because in one dimension particles cannot pass by one another as they can in higher dimensions; their mass center coordinates, satisfy the relations . As a result the configuration integral is simply .
In 1959 this one-dimensional gas model was extended by Kac to include particle pair interactions through an attractive potential, . This specific form allowed evaluation of the grand partition function,
in the thermodynamic limit, in terms of the eigenfunctions and eigenvalues of a homogeneous integral equation. Although an explicit equation of state was not obtained, it was proved that the pressure was a strictly decreasing function of the volume per particle, hence condensation did not occur.

Four years later, in 1963, Kac together with Uhlenbeck and Hemmer modified the pair potential of Kac's previous work as , so that
was independent of . They found, that a second limiting process they called the van der Waals limit, (in which the pair potential becomes both infinitely long range and infinity weak) and performed after the thermodynamic limit, produced the one-dimensional vdW equation (here rendered in molar form)
in which and , together with the Gibbs criterion, (equivalently the Maxwell construction). As a result all isotherms satisfy the condition as shown in Fig. 9, and hence the first criticism of the vdW equation is not as serious as originally thought.
Then, in 1966, Lebowitz and Penrose generalized what they called the Kac potential to apply to a non specific function in an arbitrary number, , of dimensions, . For and this reduces to the specific one-dimensional function considered by Kac, et. al. and for it is an arbitrary function (although subject to specific requirements) in physical three dimensional space. In fact the function must be bounded, non-negative, and one whose integral
is finite, independent of . By obtaining upper and lower bounds on and hence on , taking the thermodynamic limit () to obtain upper and lower bounds on the function , then subsequently taking the van der Waals limit, they found that the two bounds coalesced and thereby produced a unique limit, here written in terms of the free energy per mole and the molar volume,
The abbreviation CE stands for convex envelope; this is a function which is the largest convex function that is less than or equal to the original function. The function is the limit function when ; also here . This result is illustrated in the present context by the solid green curves and black line in Fig. 8, which is the convex envelope of also shown there.
The corresponding limit for the pressure is a generalized form of the vdW equation
together with the Gibbs criterion, (equivalently the Maxwell construction). Here is the pressure when attractive molecular forces are absent.
The conclusion from all this work is that a rigorous mathematical derivation from the partition function produces a generalization of the vdW equation together with the Gibbs criterion if the attractive force is infinitely weak with an infinitely long range. In that case the pressure that results from direct particle collisions (or more accurately the core repulsive forces), replaces . This is consistent with the second criticism that can be stated as . Consequently the vdW equation cannot be rigorously derived from the configuration integral over the entire range of .
Nevertheless, it is possible to rigorously show that the vdW equation is equivalent to a two term approximation of the virial equation, hence it can be rigorously derived from the partition function as a two term approximation in the additional limit .
The virial equation of state
This derivation is simplest when begun from the grand partition function, (see above for its definition),
In this case the connection with thermodynamics is through , together with the number of particles Substituting the expression for written above in the series for produces
expanding in its convergent power series, using the series for in each term, and equating powers of produces relations that can be solved for the in terms of the . For example , , and .
Then from , the number density, , is expressed as the series
The coefficients are given in terms of by a known formula, or determined simply by substituting into the series for , and equating powers of ; thus , etc. Finally, using this series in the series for produces the virial expansion, or virial equation of state
The second virial coefficient
This conditionally convergent series is also an asymptotic power series for the limit , and a finite number of terms is an asymptotic approximation to . The dominant order approximation in this limit is , which is the ideal gas law. It can be written as an equality using order symbols, for example , which states that the remaining terms approach zero in the limit, or , which states, more accurately, that they approach zero in proportion to . The two term approximation is , and the expression for is

where and is a dimensionless two particle potential function. For spherically symetric molecules this function can be represented most simply with two parameters, , a characteristic molecular diameter, and binding energy respectively as shown in the accompanying plot in which . Also for spherically symetric molecules 5 of the 6 integrals in the expression for can be done with the result
From its definition is positive for , and negative for with a minimum of at some . Furthermore increases so rapidly that whenever then . In addition in the limit ( is a dimensionless coldness, and the quantity is a characteristic molecular temperature) the exponential can be approximated for by two terms of its power series expansion. In these circumstances can be approximated as
where has the minimum value of . On splitting the interval of integration into 2 parts, one less than and the other greater than , evaluating the first integral, and making the second integration variable dimensionless using produces,
where and with a numerical factor whose value depends on the specific dimensionless intermolecular pair potential
Here where are the constants given in the introduction. The condition that be finite requires that be integrable over the range [1,). This result indicates that a dimensionless that is a function of a dimensionless molecular temperature is a universal function for all real gases with an intermolecular pair potential of the form ; this is an example of the principle of corresponding states on the molecular level. Moreover this is true in general and has been developed extensively both theoretically and experimentally.
The van der Waals Approximation
Substituting the (approximate in ) expression for into the two term virial approximation produces
Here the approximation is written in terms of molar quantities; its first two terms are the same as the first two terms of the vdW virial equation.
The Taylor expansion of , uniformly convergent for , can be written as , so substituting for produces
Alternatively this is
the vdW equation.
Summary
According to this derivation the vdW equation is an equivalent of the two term approximation of the virial equation of statistical mechanics in the limits . Consequently the equation produces an accurate approximation in a region defined by (on a molecular basis ), which corresponds to a dilute gas. But as the density becomes larger the behavior of the vdW approximation and the 2 term virial expansion differ markedly. Whereas the virial approximation in this instance either increases or decreases continuously, the vdW approximation together with the Maxwell construction expresses physical reality in the form of a phase change, while also indicating the existence of metastable states. This difference in behaviors was pointed out long ago by Korteweg, and Rayleigh (see Rowlinson) in the course of their dispute with Tait about the vdW equation.
In this extended region, use of the vdW equation is not justified mathematically, however it has empirical validity. Its various applications in this region that attest to this, both qualitative and quantitative, have been described previously in this article. This point was also made by Alder, et. al. who, at a conference marking the 100th anniverary of van der Waals thesis, noted that:
It is doubtful whether we would celebrate the centenial of the Van der Waals equation if it were applicable only under circumstances where it has been proven to be rigorously valid. It is empirically well established that many systems whose molecules have attractive potentials that are neither long-range nor weak conform nearly quantatively to the Van der Waals model. An example is the theoretically much studied system of Argon, where the attractive potential has only a range half as large as the repulsive core.
They continued by saying that this model has "validity down to temperatures below the critical temperature, where the attractive potential is not weak at all but, in fact, comparable to the thermal energy." They also described its application to mixtures "where the Van der Waals model has also been applied with great success. In fact, its success has been so great that not a single other model of the many proposed since, has equalled its quantitative predictions, let alone its simplicity."
Engineers have made extensive use of this empirical validity, modifying the equation in numerous ways (by one account there have been some 400 cubic equations of state produced) in order to manage the liquids, and gases of pure substances and mixtures, they encounter in practice.
This situation has been described by Boltzmann most aptly as follows:
...van der Waals has given us such a valuable tool that it would cost us much trouble to obtain by the subtlest deliberations a formula that would really be more useful than the one that van der Waals found by inspiration, as it were.
Notes
- Goodstein, pp. 303-304, 316, 452
- Jean Perrin (1909). "Le Mouvement Brownien et la Réalité Moleculaire". Annales de chimie et de physique. 18 (8è série): 5–114.
- van der Waals, (1910)
- Goodstein, pp. 443-463
- DeBoer, pp. 7-16
- Valderrama (2010), pp. 415-420
- Kontogeorgis, et al., pp. 4619-4637
- van der Waals, p. 174. sfn error: no target: CITEREFvan_der_Waals (help)
- Epstein, P.S., p 9
- Boltzmann, p 231
- van der Waals, pp. 168-172
- Boltzmann, p. 221–224
- van der Waals, p. 172
- van der Waals, (1910) p. 256
- van der Waals, p. 173
- Hirschfelder, et. al., pp. 31-34
- Hirschfelder, et. al., pp. 31-34
- Goodstein, pp. 250, 263
- Tien, Lienhard, pp. 250, 251
- Truesdell, Bharatha, pp 13–15
- Epstein, p. 11
- ^ Epstein, p.10
- Boltzmann, L. Enzykl. der Mathem. Wiss., V,(1), 550
- Sommerfeld, p 55
- ^ Sommerfeld, p 66
- Sommerfeld, pp. 55–68
- ^ Lienhard, pp. 172-173
- Peck, R.E.
- ^ Pitzer, K.S., et al., p.3433
- Goodstein, pp 443–452
- Weinberg, S., pp. 4–5
- Weinberg, p. 33
- Gibbs, J.W., pp vii–xii
- van der Waals, J.D., (1873), "Over de Continuïteit van den Gas en Vloeistoftoestand", Leiden, Ph.D. Thesis Leiden Univ
- van der Waals, (1984), pp.121–240
- Boltzmann, p 218
- Andrews, T., (1869), "On the Continuity of the Gaseous and Liquid States of Matter", Philosophical Transactions of the Royal Society of London, 159, 575-590
- Klein, M. J., p. 31
- van der Waals, pp. 125, 191–194
- Goodstein, pp. 450–451
- Boltzmann, pp. 232–233
- Goodstein, p. 452
- van der Waals, Rowlinson (ed.), p. 19
- ^ Lekner, pp.161-162
- Sommerfeld, pp. 56–57
- Goodstein, p 449
- Boltzmann, pp 237-238
- Boltzmann, pp 239–240
- Barenblatt, p. 16.
- Barenblatt, pp. 13–23
- Sommerfeld, p. 57
- Johnston, p. 6
- ^ Dong and Lienhard, pp. 158-159
- Whitman, p 155
- ^ Moran and Shapiro, p 574
- Johnston, p. 10
- ^ Johnston, p. 11
- Whitman, p. 203
- Sommerfeld, p 56
- Whitman, p. 204
- Moran and Shapiro, p. 580
- Johnston, p. 3
- ^ Johnston, p.12
- Callen, pp 131–135
- Lienhard, et al., pp. 297-298
- Callen, pp. 37–44
- Callen, p. 153
- Callen, pp. 85–101
- ^ Callen, pp. 146–156
- Maxwell, pp. 358-359
- Shamsundar and Lienhard, pp. 878,879
- Barrufet,and Eubank, pp. 170
- Johnston, D.C., pp 16-18
- Boltzmann, pp. 248–250
- Lienhard, et al., p 297
- Tien and Lienhard, p.254
- van der Waals, Rowlinson (ed.), p. 22
- Sommerfeld, pp. 61–63
- Sommerfeld, pp 60-62
- Sommerfeld, p 61
- Sommerfeld, p. 62 Fig.8
- Van Wylen and Sonntag, p. 49
- Johnston, p. 10
- Su, G.J., (1946), "Modified Law of Corresponding States for Real Gases", Ind. Eng. Chem., 38, 803
- Moran, and Shapiro, p. 113
- Tien and Lienhard, pp. 247–248
- Boltzmann, pp. 353-356
- van der Waals, Rowlinson (ed.), pp. 20-22
- van der Waals, pp. 243-282
- Lorentz, H. A., (1881), Ann. der Physik und Chemie, 12, 127, 134, 600
- van der Waals, Rowlinson (ed.), p. 68
- van der Waals, p. 244
- ^ Redlich, O.; Kwong, J. N. S. (1949). "On the Thermodynamics of Solutions. V. An Equation of State. Fugacities of Gaseous Solutions" (PDF). Chemical Reviews. 44 (1): 233–244. doi:10.1021/cr60137a013. PMID 18125401. Retrieved 2 April 2024.
- Callen, p. 105
- van der Waals, pp. 245-247
- Lebowitz, p. 52
- Kreyszig, pp. 124-128
- Callen, pp. 47-48
- van der Waals, Rowlinson (ed.), pp. 23-27
- van der Waals, pp. 253-258
- DeBoer, 7-16
- van der Waals, Rowlinson (ed.), pp. 23-27, 64-66
- van der Waals, Rowlinson (ed.), p. 66
- Hirschfelder, et al., pp. 252-253
- Hirschfelder, et al., pp. 168-169
- Hewitt, Nigel. "Who was Van der Waals anyway and what has he to do with my Nitrox fill?". Maths for Divers. Archived from the original on 11 March 2020. Retrieved 1 February 2019.
- Valderrama, pp. 1308-1312
- Kontogeorgis, et. al., pp. 4626-4633
- Niemeyer, Kyle. "Mixture properties". Computational Thermodynamics. Archived from the original on 2 April 2024. Retrieved 2 April 2024.
- van der Waals, Rowlinson (ed.), p. 69
- Leland, T. W., Rowlinson, J.S., Sather, G.A., and Watson, I.D., Trans. Faraday Soc., 65, 1447, (1968)
- van der Waals, Rowlinson (ed.), p. 69-70
- van der Waals, Rowlinson (ed.), p. 70
- Goodstein, p. 443
- Korteweg, p. 277
- Tonks, pp. 962-963
- Kac, et. al. p. 224.
- Goodstein, p. 446
- Goodstein, pp. 51, 61-68
- Tien and Lienhard, pp. 241-252
- Hirschfelder, et al., pp. 132-141
- Hill, pp. 112-119
- Hirschfelder, et. al., p. 133
- Kac, et. al., p. 223.
- Korteweg, p. 277.
- van der Waals, (1910), p.256
- van Hove, p.951
- Korteweg, p. 153.
- Rayleigh, p.81 footnote 1
- Tonks, p. 959
- Kac, p. 224
- Kac
- Kac, et. al., p216-217
- Kac, et. al., p. 224
- Lebowitz and Penrose, p.98
- Lebowitz, pp. 50-52
- Hill, pp. 24,262
- Hill, pp. 262-265
- Hinch, pp. 21-21
- Cole, pp. 1-2
- Goodstein, p. 263
- Tien, and Lienhard, p. 250
- Hill, p. 208
- Hirschfelder, et al., pp. 156-173
- Hill, pp. 270-271
- Tien, and Lienhard, p.251
- Korteweg, p.
- Rowlinson, p. 20
- Alder, et. al., P. 143
- Singer, J.V.R., and Singer, K., Mol. Phys.(1972), 24, 357; McDonald, J.R., (1972), 24, 391
- Alder, et. al., p. 144
- Valderrama, p. 1606
- Vera and Prausnitz, p. 7-10
- Kontogeorgis, et. al., pp. 4626-4629
- Boltzmann, p. 356
References
- Alder, B. J.; Alley, W. E.; Rigby, M. (1974). "Correction to the van der Waals model for mixtures and for the diffusion coefficient". Physica. 74 (1): 143–155. Bibcode:1974Phy....73..143A. doi:10.1016/0031-8914(74)90231-6.
- Barenblatt, G.I. (1979) . Similarity, Self-Similarity, and Intermediate Asymptotics. Translated by Stein, Norman. Translation Editor VanDyke, Milton. NY and London. Consultants Bureau.
- Barrufet, M.A.; Eubank, P.T. (1989). "Generalized Saturation Properties of Pure Fluids Via Cubic Equations of State". Chemical Engineering Education. 23 (3): 168–175.
- Boltzmann, L. (1995) . Lectures on Gas Theory. Translated by Brush, S.G. NY: Dover.
- Callen, H.B. (1960). Thermodynamics. NY: John Wiley ans Sons.
- DeBoer, J. (1974). "Van der Waals in his time and the present revival opening address". Physica. 73 (1): 1–27. Bibcode:1974Phy....73....1D. doi:10.1016/0031-8914(74)90223-7.
- Dong, W.G.; Lienhard, J.H. (1986). "Corresponding States Correlation of Saturated and Metastable Properties". Canad J Chem Eng. 64: 158–161. doi:10.1002/cjce.5450640123.
- Epstein, P.S. (1937). Textbook of Thermodynamics. NY: John Wiley and Sons.
- Gibbs, J.W. (1948) . The Collected Works of J. Willard Gibbs Volume II Part One Elementary Principles in Statistical Mechanics. New Haven: Yale University Press.
- Goodstein, D.L. (1985) . States of Matter. NY: Dover.
- Hill, Terrell L. (1986). Statistical Thermodynamics. NY: Dover.
- Hirschfelder, J. O.; Curtis, C. F.; Bird, R. B. (1964). Mollecular Theory of Gases and Liquids, corrected printing. NY: John Wiley and Sons, Inc.
- Johnston, D.C. (2014). Advances in Thermodynamics of the van der Waals Fluid. arXiv:1402.1205. Bibcode:2014atvd.book.....J. doi:10.1088/978-1-627-05532-1. ISBN 978-1-627-05532-1.
- Kac, Marc (1958). "On the Partition Function of a One-Dimensional Gas". Phys Fluids. 1: 8–12.
- Kac, M.; Uhlenbeck, G.E.; Hemmer, P.C. (1963). "On the van der Waals Theory of the Vapor-Liquid Equilibrium. 1. Discussion of a One-Dimensional Model". J. Math. Phys. 4 (2): 216–228. Bibcode:1963JMP.....4..216K. doi:10.1063/1.1703946.
- Kreyszig, E. (1959). Differential Geometry. Toronto: University of Toronto Press.
- Klein, M. J. (1974). "The Historical Origins of the Van der Waals Equation". Physica. 73 (1): 28–47. Bibcode:1974Phy....73...28K. doi:10.1016/0031-8914(74)90224-9.
- Kontogeorgis, G.M.; Privat, R.; Jaubert, J-N.J. (2019). "Taking Another Look at the van der Waals Equation of State---Amost 150 Years Later". J. Chem. Eng. Data. 64 (11): 4619–4637. doi:10.1021/acs.jced.9b00264.
- Korteweg, D.T. (1891). "On Van Der Waals Isothermal Equation". Nature. 45 (1155): 152–154. Bibcode:1891Natur..45..152K. doi:10.1038/045152a0.
- Korteweg, D.T. (1891). "On Van Der Waals Isothermal Equation". Nature. 45 (1160): 277. doi:10.1038/045277a0.
- Lebowitz, J.L. (1974). "Exact Derivation of the Van Der Waals Equation". Physica. 73 (1): 48–60. Bibcode:1974Phy....73...48L. doi:10.1016/0031-8914(74)90225-0.
- Lebowitz, J.L.; Penrose, O. (1966). "Rigorous Treatment of the Van der Waals-Maxwell Theory of the Liquid-Vapor Transition". Jour Math Phys. 7 (1): 98–113. Bibcode:1966JMP.....7...98L. doi:10.1063/1.1704821.
- Lekner, J. (1982). "Parametric solution of the van der Waals liquid–vapor coexistence curve". Am. J. Phys. 50 (2): 161–163. Bibcode:1982AmJPh..50..161L. doi:10.1119/1.12877.
- Lienhard, J.H. (1986). "The Properties and Behavior of Superheated Liquids". Lat. Am. J. Heat and Mass Transfer. 10: 169–187.
- Lienhard, J.H; Shamsundar, N.; Biney, P.O. (1986). "Spinodal Lines and Equations of State: A Review". Nuclear Engineering and Design. 95: 297–314. Bibcode:1986NuEnD..95..297L. doi:10.1016/0029-5493(86)90056-7.
- Maxwell, J.C. (1875). "On the Dynamical Evidence of the Molecular Constitution of Bodies". Nature. 11 (279): 357–359. Bibcode:1875Natur..11..357C. doi:10.1038/011357a0.
- Moran, M.J.; Shapiro, H.N. (2000). Fundamentals of Engineering Thermodynamics 4th Edition. NY: McGraw-Hill.
- Peck, R.E. (1982). "The Assimilation of van der Waals Equation in the Corresponding States Family". Can. J. Chem. Eng. 60: 446–449. doi:10.1002/cjce.5450600319.
- Pitzer, K.S.; Lippman, D.Z.; Curl, R.F.; Huggins, C.M.; Peterson, D.E. (1955). "The Volumetric and Thermodynamic Properties of Fluids. II. Compressibility Factor, Vapor Pressure and Entropy of Vaporization". J. Am. Chem. Soc. 77 (13): 3433–3440. Bibcode:1955JAChS..77.3433P. doi:10.1021/ja01618a002.
- Shamsundar, N.; Lienhard, J.H. (1983). "Saturation and Metastable Properties of the van der Waals Fluid". Canad J Chem Eng. 61 (6): 876–880. doi:10.1002/cjce.5450610617.
- Sommerfeld, A. (1956). Bopp, F.; Meixner, J. (eds.). Thermodynamics and Statistical Mechanics - Lectures on Theoretical Physics Volume V. Translated by Kestin, J. NY: Academic Press.
- Strutt, J.W., 3rd Baron Rayleigh (1891). "On the Virial of a System of Hard Colliding Bodies". Nature. 45 (1152): 80–82. Bibcode:1891Natur..45...80R. doi:10.1038/045080a0.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link)
- Tien, C.L.; Lienhard, J.H. (1979). Statistical Thermodynamics Revised Printing. NY: Hemisphere Publishing. Bibcode:1979wdch.book.....T.
- Tonks, L. (1936). "The Complete Equation of State of One, Two, and Three-Dimensional Gases of Hard Elastic Spheres". Phys. Rev. 50 (10): 955–963. Bibcode:1936PhRv...50..955T. doi:10.1103/PhysRev.50.955.
- Truesdell, C.; Bharatha, S. (1977). Classical Thermodynamics as a Theory of Heat Engines. NY: Springer-Verlag.
- Valderrama, J.O. (2003). "The State of the Cubic Equations of State". Ind. Chem. Eng. Res. 42 (8): 1603–1618. doi:10.1021/ie020447b.
- van der Waals, J.D. (1984). Rowlinson, J.S. (ed.). On the Continuity of the Gaseous and Liquid States, edited and with an Introduction by J.S. Rowlinson. NY: Dover Phoenix Editions.
- van der Waals, Johannes D. (1967). "The Equation of State of Gases and Liquids". in Nobel Lectures, Physics 1901-1921. Amsterdam: Elsevier. pp. 254–265.
- van Hove, L. (1949). "Quelques Proprieties Generales De L'Integrale De Configuration D'Un Systeme De Particules Avec Interaction". Physica. 15 (11–12): 951–961. Bibcode:1949Phy....15..951V. doi:10.1016/0031-8914(49)90059-2.
- Van Wylen, G.J.; Sonntag, R.E. (1973). Fundamentals of Classical Thermodynamics Second Edition. NY: John Wiley ans Sons.
- Vera, J.H.; Prausnitz, J.M. (1972). "Generalized van der Waals Theory for Dense Fluids". Chem. Eng. Jour. 3: 1–13. doi:10.1016/0300-9467(72)85001-9.
- Weinberg, S. (2021). Foundations of Modern Physics. Cambridge: Cambridge University Press.
- Whitman, A.M. (2023). Thermodynamics: Basic Principles and Engineering Applications 2nd Edition. NY: Springer.
See also
- Gas laws
- Ideal gas
- Inversion temperature
- Iteration
- Maxwell construction
- Real gas
- Theorem of corresponding states
- Van der Waals constants (data page)
- Redlich–Kwong equation of state
Further reading
- Chandler, David (1987). Introduction to Modern Statistical Mechanics. Oxford: Oxford University Press. pp. 287–295. ISBN 0195042778.
- Cross, Michael (2004), "Lecture 3: First Order Phase Transitions" (PDF), Physics 127: Statistical Physics, Second Term, Pasadena, California: Division of Physics, Mathematics, and Astronomy, California Institute of Technology.
- Dalgarno, A.; Davison, W.D. (1966). "The Calculation of Van Der Waals Interactions". Advances in Atomic and Molecular Physics. 2: 1–32. Bibcode:1966AdAMP...2....1D. doi:10.1016/S0065-2199(08)60216-X. ISBN 9780120038022.
- Kittel, Charles; Kroemer, Herbert (1980). Thermal Physics (Revised ed.). New York: Macmillan. pp. 287–295. ISBN 0716710889.
 , appears at the location (1,1,1) of the 3 dimensional plot space. This device makes it easy to compare this surface with the one generated by the van der Waals equation in Fig. C. Figures A and C are drawn with the same scales and limits; they also present the two surfaces from the same viewpoint to make the comparison easier. Whereas the ideal gas surface is rather plain, the van der Waals surface has an interesting fold.
, appears at the location (1,1,1) of the 3 dimensional plot space. This device makes it easy to compare this surface with the one generated by the van der Waals equation in Fig. C. Figures A and C are drawn with the same scales and limits; they also present the two surfaces from the same viewpoint to make the comparison easier. Whereas the ideal gas surface is rather plain, the van der Waals surface has an interesting fold.

 is pressure,
is pressure,  is temperature, and
is temperature, and  is molar volume. In addition
is molar volume. In addition  is the
is the  is the
is the  is the number of molecules (the ratio
is the number of molecules (the ratio  is a
is a  is the
is the  is the
is the  and
and  are experimentally determinable, substance-specific constants.
are experimentally determinable, substance-specific constants.
 , would come into contact when their centers were a distance
, would come into contact when their centers were a distance  about the other. That is 8 times
about the other. That is 8 times  , the volume of each particle of radius
, the volume of each particle of radius  , but there are 2 particles which gives 4 times the volume per particle. The total excluded volume is then
, but there are 2 particles which gives 4 times the volume per particle. The total excluded volume is then  , 4 times the volume of all the particles. Van der Waals and his contemporaries used an alternative, but equivalent, analysis based on the mean free path between molecular collisions that gave this result. From the fact that the volume fraction of particles,
, 4 times the volume of all the particles. Van der Waals and his contemporaries used an alternative, but equivalent, analysis based on the mean free path between molecular collisions that gave this result. From the fact that the volume fraction of particles,  must be positive, van der Waals noted that as
must be positive, van der Waals noted that as  ), but he was never able to determine the nature of the decrease. The constant
), but he was never able to determine the nature of the decrease. The constant  in the equation above has
in the equation above has  (with
(with  ). Two of the many such functions that have been suggested are shown in the accompanying plot.
). Two of the many such functions that have been suggested are shown in the accompanying plot.
 obtained by van der Waals and his contemporaries. This result is valid for any pair potential for which the increase in
obtained by van der Waals and his contemporaries. This result is valid for any pair potential for which the increase in  is sufficiently rapid. This includes the hard sphere model for which the increase is infinitely rapid and the result is exact. Indeed the Sutherland potential most accurately models van der Waals' conception of a molecule. It also includes potentials that do not represent hard sphere force interactions provided that the increase in
is sufficiently rapid. This includes the hard sphere model for which the increase is infinitely rapid and the result is exact. Indeed the Sutherland potential most accurately models van der Waals' conception of a molecule. It also includes potentials that do not represent hard sphere force interactions provided that the increase in  is fast enough, but then it is approximate; increasingly better the faster the increase. In that case
is fast enough, but then it is approximate; increasingly better the faster the increase. In that case  where
where  is a number that depends on the shape of the potential function,
is a number that depends on the shape of the potential function,  . However, this result is only valid when the potential is weak, namely, when the minimum potential energy is very much smaller than the thermal energy,
. However, this result is only valid when the potential is weak, namely, when the minimum potential energy is very much smaller than the thermal energy,  .
.
 (
( (molar volume) used here,
(molar volume) used here,  the reciprocal of
the reciprocal of  , those using specific volume contain
, those using specific volume contain  (the substance specific
(the substance specific  is the
is the  the mass of a single particle), and those written with number density contain
the mass of a single particle), and those written with number density contain  ,
,  such that the substance cannot be liquefied either when
such that the substance cannot be liquefied either when  no matter how low the temperature, or when
no matter how low the temperature, or when  no matter how high the pressure;
no matter how high the pressure;  uniquely define
uniquely define  ), and other attributes. These predictions are accurate for only a few substances. For most simple fluids they are only a valuable approximation. The equation also explains why
), and other attributes. These predictions are accurate for only a few substances. For most simple fluids they are only a valuable approximation. The equation also explains why  , and vapor,
, and vapor,  , at
, at  . The locus of the saturated liquid and vapor states--the green dots in this figure--for all subcritical isobars form the saturation curve on the
. The locus of the saturated liquid and vapor states--the green dots in this figure--for all subcritical isobars form the saturation curve on the  surface.
surface. plane corresponding to the value of the pressure chosen.
plane corresponding to the value of the pressure chosen.
 , the slope is positive over the entire range,
, the slope is positive over the entire range,  (although the plot only shows a finite quadrant). This describes a fluid as
(although the plot only shows a finite quadrant). This describes a fluid as 
 , has a region of negative slope. This region consists of states that are unstable and therefore never observed (for this reason this region is shown dotted gray). The green curve thus consists of two disconnected branches; a
, has a region of negative slope. This region consists of states that are unstable and therefore never observed (for this reason this region is shown dotted gray). The green curve thus consists of two disconnected branches; a  , an average of
, an average of  , a stable liquid for
, a stable liquid for  , and a mixture of liquid and vapor at
, and a mixture of liquid and vapor at  , that also supports metastable states of subcooled vapor and superheated liquid. It is characteristic of all subcritical isobars
, that also supports metastable states of subcooled vapor and superheated liquid. It is characteristic of all subcritical isobars  , where
, where  is a function of
is a function of  , is sufficiently small), Specifically
, is sufficiently small), Specifically
 , then
, then  is numerically indistinguishable from
is numerically indistinguishable from  , then
, then  is numerically indistinguishable from
is numerically indistinguishable from 
 , can be shown to be a function of
, can be shown to be a function of  , entropy
, entropy  , as well as the specific heat at constant pressure
, as well as the specific heat at constant pressure  have simple analytic expressions [this is also true of enthalpy
have simple analytic expressions [this is also true of enthalpy  , Helmholtz free energy
, Helmholtz free energy  , and Gibbs free energy
, and Gibbs free energy  ]
] has a simple analytic expression [this is also true of its isothermal compressibility,
has a simple analytic expression [this is also true of its isothermal compressibility,  ]
] , or the
, or the  , where
, where  is a dimensionless saturation pressure, and log is the logarithm base 10. Consequently, the equation plays an important role in the modern theory of phase transitions.
is a dimensionless saturation pressure, and log is the logarithm base 10. Consequently, the equation plays an important role in the modern theory of phase transitions.
 for the pressure,
for the pressure,  , mass
, mass  . He then noted that using the classical laws of Boyle and Charles one could write
. He then noted that using the classical laws of Boyle and Charles one could write  with
with  CO
CO , that showed at low temperatures a jump in density at a certain pressure, while at higher temperatures there was no abrupt change; the figure can be seen
, that showed at low temperatures a jump in density at a certain pressure, while at higher temperatures there was no abrupt change; the figure can be seen  and
and  in two states with the same density, the van der Waals equation produces the values,
in two states with the same density, the van der Waals equation produces the values,

 (the parameter
(the parameter  is related to the entropy difference between the two phases), that was first obtained by Plank, was known to Gibbs and others, and was later derived in a beautifully simple and elegant manner by Lekner. A summary of Lekner's solution is presented in a subsequent section, and a more complete discussion in the
is related to the entropy difference between the two phases), that was first obtained by Plank, was known to Gibbs and others, and was later derived in a beautifully simple and elegant manner by Lekner. A summary of Lekner's solution is presented in a subsequent section, and a more complete discussion in the  the slope is negative,
the slope is negative,  , everywhere except at a single point, the critical point,
, everywhere except at a single point, the critical point,  , where both the slope and curvature are zero,
, where both the slope and curvature are zero, 
 for which the equation has 1 real root for
for which the equation has 1 real root for  , and using these in the equation gives
, and using these in the equation gives  .
.


 , whose solution produces the previous results for
, whose solution produces the previous results for  .
.
 renders the equation in the dimensionless form used to construct Fig. 1
renders the equation in the dimensionless form used to construct Fig. 1

 will plot on the same curve. It expresses the law of corresponding states which Boltzmann described as follows:
will plot on the same curve. It expresses the law of corresponding states which Boltzmann described as follows:
 , and 3 independent dimensions, , , (independent means that "none of the dimensions of these quantities can be represented as a product of powers of the dimensions of the remaining quantities", and =), must be expressible in terms of 6 − 3 = 3 dimensionless groups. Here
, and 3 independent dimensions, , , (independent means that "none of the dimensions of these quantities can be represented as a product of powers of the dimensions of the remaining quantities", and =), must be expressible in terms of 6 − 3 = 3 dimensionless groups. Here  is a characteristic molar volume,
is a characteristic molar volume,  a characteristic pressure, and
a characteristic pressure, and  a characteristic temperature, and the 3 dimensionless groups are
a characteristic temperature, and the 3 dimensionless groups are  . According to dimensional analysis the equation must then have the form
. According to dimensional analysis the equation must then have the form  , a general similarity relation. In his discussion of the vdW equation Sommerfeld also mentioned this point. The reduced properties defined previously are
, a general similarity relation. In his discussion of the vdW equation Sommerfeld also mentioned this point. The reduced properties defined previously are  ,
,  , and
, and  . Recent research has suggested that there is a family of equations of state that depend on an additional dimensionless group, and this provides a more exact correlation of properties. Nevertheless, as Boltzmann observed, the van der Waals equation provides an essentially correct description.
. Recent research has suggested that there is a family of equations of state that depend on an additional dimensionless group, and this provides a more exact correlation of properties. Nevertheless, as Boltzmann observed, the van der Waals equation provides an essentially correct description.
 , while for most real fluids
, while for most real fluids  . Thus most real fluids do not satisfy this condition, and consequently their behavior is only described qualitatively by the vdW equation. However, the vdW equation of state is a member of a family of state equations based on the Pitzer (
. Thus most real fluids do not satisfy this condition, and consequently their behavior is only described qualitatively by the vdW equation. However, the vdW equation of state is a member of a family of state equations based on the Pitzer ( , and the liquid metals, Mercury and Cesium, are well approximated by it.
, and the liquid metals, Mercury and Cesium, are well approximated by it.
 , and a constant volume specific heat,
, and a constant volume specific heat,  .
.

 is an arbitrary constant of integration.
is an arbitrary constant of integration.
 to be an exact differential, namely that
to be an exact differential, namely that  be continuous with continuous partial derivatives, its second mixed partial derivatives must also be equal,
be continuous with continuous partial derivatives, its second mixed partial derivatives must also be equal,  . Then with
. Then with  this condition can be written simply as
this condition can be written simply as  . Differentiating
. Differentiating  for the vdW equation gives
for the vdW equation gives ![{\displaystyle T^{2}\partial _{T}(p/T)]=a/v^{2}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ed657ecc07611888aa01279750d8d307efb713a8) , so
, so  . Consequently
. Consequently  for a vdW fluid exactly as it is for an ideal gas. To keep things simple it is regarded as a constant here,
for a vdW fluid exactly as it is for an ideal gas. To keep things simple it is regarded as a constant here,  with
with  a number. Then both integrals can be easily evaluated and the result is
a number. Then both integrals can be easily evaluated and the result is


 and
and  is a dimensionless constant.
is a dimensionless constant.
 is just
is just  . Then
. Then
 is simply
is simply


 we obtain
simply
we obtain
simply




 so combining the previous results gives
so combining the previous results gives



 , the isothermal compressibility, is a measure of the relative increase of volume from an increase of pressure, at constant temperature, while
, the isothermal compressibility, is a measure of the relative increase of volume from an increase of pressure, at constant temperature, while  , the coefficient of thermal expansion, is a measure of the relative increase of volume from an increase of temperature, at constant pressure. Therefore,
, the coefficient of thermal expansion, is a measure of the relative increase of volume from an increase of temperature, at constant pressure. Therefore,


 while
while  . Since the vdW equation in this limit becomes
. Since the vdW equation in this limit becomes  , finally
, finally  . Both of these are the ideal gas values, which is consistent because, as noted earlier, the vdW fluid behaves like an ideal gas in this limit.
. Both of these are the ideal gas values, which is consistent because, as noted earlier, the vdW fluid behaves like an ideal gas in this limit.
 . However, it is not independent of
. However, it is not independent of  . Then the two partials of the vdW equation can be used to express
. Then the two partials of the vdW equation can be used to express 
 , which is also the ideal gas result as expected; however the limit
, which is also the ideal gas result as expected; however the limit  gives the same result, which does not agree with experiments on liquids.
gives the same result, which does not agree with experiments on liquids.
 , namely that the vdW liquid is incompressible. Moreover, since
, namely that the vdW liquid is incompressible. Moreover, since  , it is also mechanically incompressible, that is
, it is also mechanically incompressible, that is  faster than
faster than  .
.
 , and
, and  are all infinite on the curve
are all infinite on the curve  . This curve, called the
. This curve, called the  , and is discussed at length in the next section.
, and is discussed at length in the next section.
 and
and  , namely that at equilibrium the entropy is a maximum. This leads to a requirement that
, namely that at equilibrium the entropy is a maximum. This leads to a requirement that  which lie between the local minimum,
which lie between the local minimum,  , and local maximum,
, and local maximum,  , for which
, for which  (shown dashed gray in Fig. 1), are unstable and thus not observed. This is the genesis of the phase change; there is a range
(shown dashed gray in Fig. 1), are unstable and thus not observed. This is the genesis of the phase change; there is a range  , for which no observable states exist. The states for
, for which no observable states exist. The states for  are liquid, and for
are liquid, and for  are vapor; the denser liquid lies below the vapor due to gravity. The transition points, states with zero slope, are called spinodal points. Their locus is the spinodal curve that separates the regions of the plane for which liquid, vapor, and gas exist from a region where no observable homogeneous states exist. This spinodal curve is obtained here from the vdW equation by differentiation (or equivalently from
are vapor; the denser liquid lies below the vapor due to gravity. The transition points, states with zero slope, are called spinodal points. Their locus is the spinodal curve that separates the regions of the plane for which liquid, vapor, and gas exist from a region where no observable homogeneous states exist. This spinodal curve is obtained here from the vdW equation by differentiation (or equivalently from  ) as
) as

 shown in Fig. 1) is the origin of the phase change, the spinodal points do not represent its full extent, because both states, saturated liquid and saturated vapor coexist in equlilbrium; they both must have the same pressure as well as the same temperature. Thus the phase change is characterized, at temperature
shown in Fig. 1) is the origin of the phase change, the spinodal points do not represent its full extent, because both states, saturated liquid and saturated vapor coexist in equlilbrium; they both must have the same pressure as well as the same temperature. Thus the phase change is characterized, at temperature  that lies between that of the minimum and maximum spinodal points, and with molar volumes of liquid,
that lies between that of the minimum and maximum spinodal points, and with molar volumes of liquid,  and vapor
and vapor  . Then from the vdW equation applied to these saturated liquid and vapor states
. Then from the vdW equation applied to these saturated liquid and vapor states

 , so another equation is required in order to specify the values of 3 of these variables uniquely in terms of a fourth. Such an equation is provided here by the equality of the Gibbs free energy in the saturated liquid and vapor states,
, so another equation is required in order to specify the values of 3 of these variables uniquely in terms of a fourth. Such an equation is provided here by the equality of the Gibbs free energy in the saturated liquid and vapor states,  . This condition of material equilibrium can be obtained from a simple physical argument as follows: the
. This condition of material equilibrium can be obtained from a simple physical argument as follows: the  , and from the first law at constant pressure
, and from the first law at constant pressure  . Equating these two, rearranging, and recalling that
. Equating these two, rearranging, and recalling that  . Integrating this over an isotherm from
. Integrating this over an isotherm from  to
to  , noting that the pressure is the same at each endpoint, and setting the result to zero yields
, noting that the pressure is the same at each endpoint, and setting the result to zero yields

 (this can be visualized most easily by imagining Fig. 1 rotated
(this can be visualized most easily by imagining Fig. 1 rotated  ); the result is a special case of material equilibrium. The last equality, which follows from integrating
); the result is a special case of material equilibrium. The last equality, which follows from integrating  , is the Maxwell
, is the Maxwell  be equal to the lower one. This form means that the thermodynamic restriction that fixes
be equal to the lower one. This form means that the thermodynamic restriction that fixes  is specified by the equation of state itself,
is specified by the equation of state itself, 

 where
where

 plane. At every point in the region between the two curves there are two states, one stable and another metastable. The metastable states,
plane. At every point in the region between the two curves there are two states, one stable and another metastable. The metastable states,  is given physically by
is given physically by  . The values of all other property discontinuities across the saturation curve also follow from this solution. These functions define the coexistence curve which is the locus of the saturated liquid and saturated vapor states of the vdW fluid. The curve is plotted in Fig. 1 and Fig. 2, two projections of the state surface. These curves and the numerical results referenced earlier agree exactly, as they must.
. The values of all other property discontinuities across the saturation curve also follow from this solution. These functions define the coexistence curve which is the locus of the saturated liquid and saturated vapor states of the vdW fluid. The curve is plotted in Fig. 1 and Fig. 2, two projections of the state surface. These curves and the numerical results referenced earlier agree exactly, as they must.
 are discontinuous. Considering
are discontinuous. Considering  of Fig. 1). He concluded that such liquid states of tensile stresses were real, as did
of Fig. 1). He concluded that such liquid states of tensile stresses were real, as did 
 is the mole fraction of the vapor. This equation is called the lever rule and applies to other properties as well. The states it represents form a horizontal line connecting the same colored dots on an isotherm, but not shown in Fig. 1 as noted already since it is a distinct equation of state for the heterogeneous combination of liquid and vapor components.
is the mole fraction of the vapor. This equation is called the lever rule and applies to other properties as well. The states it represents form a horizontal line connecting the same colored dots on an isotherm, but not shown in Fig. 1 as noted already since it is a distinct equation of state for the heterogeneous combination of liquid and vapor components.
 . However, as Boltzmann noted, such a simple representation could not correctly describe all substances. Indeed, the saturation analysis of this form produces
. However, as Boltzmann noted, such a simple representation could not correctly describe all substances. Indeed, the saturation analysis of this form produces  , namely all substances have the same dimensionless coexistence curve. In order to avoid this paradox an extended principle of corresponding states has been suggested in which
, namely all substances have the same dimensionless coexistence curve. In order to avoid this paradox an extended principle of corresponding states has been suggested in which  where
where  is a substance dependent dimensionless parameter related to the only physical feature associated with an individual substance, its critical point.
is a substance dependent dimensionless parameter related to the only physical feature associated with an individual substance, its critical point.
 , is more useful. The saturation pressure in this situation is represented by a one parameter family of curves,
, is more useful. The saturation pressure in this situation is represented by a one parameter family of curves,  . Several investigators have produced correlations of saturation data for a number of substances, the best is that of Dong and Lienhard,
. Several investigators have produced correlations of saturation data for a number of substances, the best is that of Dong and Lienhard,


 over the range
over the range 
 vs
vs  . This plot shows clearly that the vdW fluid (
. This plot shows clearly that the vdW fluid ( ) is a member of the class of real fluids; indeed it quantitatively describes the behavior of the liquid metals cesium (
) is a member of the class of real fluids; indeed it quantitatively describes the behavior of the liquid metals cesium ( ) and mercury (
) and mercury ( ) whose values of
) whose values of  , is of practical importance because the two end states of a throttling process (
, is of practical importance because the two end states of a throttling process ( ) lie on a constant enthalpy curve. Although ideal gases, for which
) lie on a constant enthalpy curve. Although ideal gases, for which  , do not change temperature in such a process, real gases do, and it is important in applications to know whether they heat up or cool down.
, do not change temperature in such a process, real gases do, and it is important in applications to know whether they heat up or cool down.

 is positive the gas temperature decreases when it passes through a throttle, and if it is negative the temperature increases. Therefore the condition
is positive the gas temperature decreases when it passes through a throttle, and if it is negative the temperature increases. Therefore the condition  defines a curve that separates the region of the
defines a curve that separates the region of the  plane where
plane where  from the region where it is less than zero. This curve is called the inversion curve, and its equation is
from the region where it is less than zero. This curve is called the inversion curve, and its equation is  . Using the expression for
. Using the expression for 
 or in terms of the critical temperature
or in terms of the critical temperature  . As Sommerfeld noted, "This is the case with air and with most other gases. Air can be cooled at will by repeated expansion and can finally be liquified."
. As Sommerfeld noted, "This is the case with air and with most other gases. Air can be cooled at will by repeated expansion and can finally be liquified."
 the equation has a simple positive solution
the equation has a simple positive solution  which, for
which, for  produces,
produces,  .
Using this to eliminate
.
Using this to eliminate 
 have been replaced by
have been replaced by  .
.
 , for
, for

 , or
, or  , and the corresponding
, and the corresponding  . The zeros of the curve
. The zeros of the curve  , are, making use of the quadratic formula,
, are, making use of the quadratic formula,  , or
, or  and
and  (
( and
and  ). In terms of the dimensionless variables,
). In terms of the dimensionless variables,  the zeros are at
the zeros are at  and
and  , while the maximum is
, while the maximum is  , and occurs at
, and occurs at  . A plot of the curve is shown in green in Fig. 5. Sommerfeld also displays this plot, together with a curve drawn using experimental data from H2. The two curves agree qualitatively, but not quantitatively. For example the maximum on these two curves differ by about 40% in both magnitude and location.
. A plot of the curve is shown in green in Fig. 5. Sommerfeld also displays this plot, together with a curve drawn using experimental data from H2. The two curves agree qualitatively, but not quantitatively. For example the maximum on these two curves differ by about 40% in both magnitude and location.
 , which has zero slope at the origin is plotted and the isotherm
, which has zero slope at the origin is plotted and the isotherm  . The abscissa here is
. The abscissa here is  which varies from 0 to 1.
which varies from 0 to 1. . Here
. Here  , called the compressibility factor, is expressed either as
, called the compressibility factor, is expressed either as  or
or  . In either case
. In either case

 , so for a van der Waals fluid the compressibility factor is simply
, so for a van der Waals fluid the compressibility factor is simply  , or in terms of reduced variables
, or in terms of reduced variables

 . At the critical point,
. At the critical point,  ,
,  .
.
 ,
,  ; the fluid behaves like an ideal gas, a point noted several times earlier. The derivative
; the fluid behaves like an ideal gas, a point noted several times earlier. The derivative  is never negative when
is never negative when  , namely when
, namely when  (
( ). Alternatively when
). Alternatively when  the initial slope is negative, it becomes zero at
the initial slope is negative, it becomes zero at  , and is positive for larger
, and is positive for larger  (see Fig. 6). In this case the value of
(see Fig. 6). In this case the value of  . Here
. Here  is called the Boyle temperature. It varies between
is called the Boyle temperature. It varies between  , and denotes a point in
, and denotes a point in  space where the equation of state reduces to the ideal gas law. However the fluid does not behave like an ideal gas there, because neither its derivatives
space where the equation of state reduces to the ideal gas law. However the fluid does not behave like an ideal gas there, because neither its derivatives  reduce to their ideal gas values, other than where
reduce to their ideal gas values, other than where  the actual ideal gas region.
the actual ideal gas region.
 vs
vs  . Also shown are the spinodal and coexistence curves described previously. The subcritical isotherm consists of stable, metastable, and unstable segments, and are identified the same as they were in Fig. 1. Also included are the zero initial slope isotherm and the one corresponding to infinite temperature.
. Also shown are the spinodal and coexistence curves described previously. The subcritical isotherm consists of stable, metastable, and unstable segments, and are identified the same as they were in Fig. 1. Also included are the zero initial slope isotherm and the one corresponding to infinite temperature.
 vs
vs  using
using 
 are the virial coefficients; the
are the virial coefficients; the  in the compressibility factor of the vdW equation in its infinite series, convergent for
in the compressibility factor of the vdW equation in its infinite series, convergent for  , produces
, produces

 is
is
+\sum _{k=2}^{\infty }\,(\rho _{r}/3)^{k-1}.}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ec91712850e9f83f962842ba5f7e16dfea94576d)
 . Notice that it can be positive or negative depending on whether or not
. Notice that it can be positive or negative depending on whether or not  , which agrees with the result found previously by differentiation.
, which agrees with the result found previously by differentiation.
 , the vdW virial expansion becomes simply
, the vdW virial expansion becomes simply

 . Boltzmann calculated its correct value as
. Boltzmann calculated its correct value as  , and used the result to propose an enhanced version of the vdW equation
, and used the result to propose an enhanced version of the vdW equation

 , this produced the correct coefficients thru
, this produced the correct coefficients thru  and also gave infinite pressure at
and also gave infinite pressure at  , which is approximately the close packing distance for hard spheres. This was one of the first of many equations of state proposed over the years that attempted to make quantitative improvements to the remarkably accurate explanations of real gas behavior produced by the vdW equation.
, which is approximately the close packing distance for hard spheres. This was one of the first of many equations of state proposed over the years that attempted to make quantitative improvements to the remarkably accurate explanations of real gas behavior produced by the vdW equation.

 where
where

 , and
, and  , with
, with  (so that
(so that  ) are the mole fractions of the two fluid substances. Adding the equations for the two fluids shows that
) are the mole fractions of the two fluid substances. Adding the equations for the two fluids shows that  , although for
, although for  with equality holding in the ideal gas limit. The quadratic forms for
with equality holding in the ideal gas limit. The quadratic forms for  and
and  are a consequence of the forces between molecules. This was first shown by Lorentz, and was credited to him by van der Waals. The quantities
are a consequence of the forces between molecules. This was first shown by Lorentz, and was credited to him by van der Waals. The quantities  and
and  in these expressions characterize collisions between two molecules of the same fluid component while
in these expressions characterize collisions between two molecules of the same fluid component while  and
and  represent collisions between one molecule of each of the two different component fluids. This idea of van der Waals was later called a one fluid model of mixture behavior.
represent collisions between one molecule of each of the two different component fluids. This idea of van der Waals was later called a one fluid model of mixture behavior.
 is the arithmetic mean of
is the arithmetic mean of  and
and  ,
,  , substituting into the quadratic form, and noting that
, substituting into the quadratic form, and noting that 
 ,
,  and
and  , namely at equilibrium the Helmholtz potential is a minimimum. Since, like
, namely at equilibrium the Helmholtz potential is a minimimum. Since, like  , the molar Helmholtz function
, the molar Helmholtz function  is also a potential function whose differential is
is also a potential function whose differential is

 . This condition means that the function,
. This condition means that the function,  , is
, is  . Thus when
. Thus when  are constant along with temperature the function
are constant along with temperature the function  represents a straight line with slope
represents a straight line with slope  , and intercept
, and intercept  , the curve and the straight line will have a single tangent. However, for a subcritical
, the curve and the straight line will have a single tangent. However, for a subcritical  is not everwhere convex. With
is not everwhere convex. With  and a suitable value of
and a suitable value of  , and saturated vapor,
, and saturated vapor,  ; there will be a double tangent. Furthermore, each of these points is characterized by the same value of
; there will be a double tangent. Furthermore, each of these points is characterized by the same value of  These are the same three specifications for coexistence that were used
These are the same three specifications for coexistence that were used  (solid-dashed green, dotted gray) at the two points
(solid-dashed green, dotted gray) at the two points  and
and  . The slope of the straight line, given by
. The slope of the straight line, given by  , is
, is  corresponding to
corresponding to  . All this is consistent with the data of the green curve,
. All this is consistent with the data of the green curve,  (
( ) continually decreases. The point characterized by
) continually decreases. The point characterized by  , is a spinodal point, and between these two points is the metastable superheated liquid. For further increases in
, is a spinodal point, and between these two points is the metastable superheated liquid. For further increases in  is the vapor. In this region the curvature continually decreases until it is zero at infinitely large
is the vapor. In this region the curvature continually decreases until it is zero at infinitely large  where
where  . Its derivative is
. Its derivative is

 , whose slope at each point is specified by the vdW equation, for the subcritical isotherm
, whose slope at each point is specified by the vdW equation, for the subcritical isotherm  , where
, where  is a composition variable, for example
is a composition variable, for example  so
so  . In this case there are three stability conditions
. In this case there are three stability conditions

 specifies the spinodal curves on the surface.
specifies the spinodal curves on the surface.

 are the molar
are the molar  . For
. For  and
and  , all constant this is the equation of a plane with slopes
, all constant this is the equation of a plane with slopes  in the
in the  must be known. In addition, the effect of collisions between molecules of the different components, given by
must be known. In addition, the effect of collisions between molecules of the different components, given by  and
and 

 fluid components, and using these empirical combinig laws, the quadradic mixing rules for the material constants are:
fluid components, and using these empirical combinig laws, the quadradic mixing rules for the material constants are:




 , which are related to
, which are related to  through
through  by
by  and
and  . Using these together with the quadratic mixing rules for
. Using these together with the quadratic mixing rules for 
 , namely for molecules of not too different diameters. In fact Rowlinson said of this approximation, "It was, and indeed still is, hard to improve on the original van der Waals recipe when expressed in form".
, namely for molecules of not too different diameters. In fact Rowlinson said of this approximation, "It was, and indeed still is, hard to improve on the original van der Waals recipe when expressed in form".
 , of statistical mechanics for a three dimensional
, of statistical mechanics for a three dimensional  Here
Here  ,
,  is the DeBroglie wavelength (alternatively
is the DeBroglie wavelength (alternatively  is the quantum concentration),
is the quantum concentration),  is the
is the  is the intermolecular potential energy, which is a function of the
is the intermolecular potential energy, which is a function of the  . Lastly
. Lastly  is the volume element of
is the volume element of  , which is a
, which is a  dimensional space.
dimensional space.
 with thermodynamics is made through the Helmholtz free energy,
with thermodynamics is made through the Helmholtz free energy,  from which all other properties can be found; in particular
from which all other properties can be found; in particular  . For point particles that have no force interactions,
. For point particles that have no force interactions,  , all
, all  can be evaluated producing
can be evaluated producing  . In the thermodynamic limit,
. In the thermodynamic limit,  with
with  finite, the Helmholtz free energy per particle (or per mole, or per unit mass) is finite, for example per mole it is
finite, the Helmholtz free energy per particle (or per mole, or per unit mass) is finite, for example per mole it is  . The thermodynamic state equations in this case are those of a monatomic ideal gas, specifically
. The thermodynamic state equations in this case are those of a monatomic ideal gas, specifically

 ; 2) the constant
; 2) the constant  in the vdw equation (here
in the vdw equation (here  , or a close packing density of 1/4=0.25, whereas the known
, or a close packing density of 1/4=0.25, whereas the known  . Thus a single value of
. Thus a single value of  are molar quantities). In addition its derivative exists and defines the pressure, which is a non increasing function of the volume per particle. Since the vdW equation has states for which the pressure increases with increasing volume per particle, this proof means it cannot be derived from the partition function, without an additional constraint that precludes those states.
are molar quantities). In addition its derivative exists and defines the pressure, which is a non increasing function of the volume per particle. Since the vdW equation has states for which the pressure increases with increasing volume per particle, this proof means it cannot be derived from the partition function, without an additional constraint that precludes those states.
 , constrained to move along a straight line of length
, constrained to move along a straight line of length  , and exerting only direct contact forces on one another satisfy a vdW equation with
, and exerting only direct contact forces on one another satisfy a vdW equation with  This can be put in a more recognizable, molar, form by dividing by the rod cross sectional area
This can be put in a more recognizable, molar, form by dividing by the rod cross sectional area  , and defining
, and defining  . This produces
. This produces  ; clearly there is no condensation,
; clearly there is no condensation,  for all
for all  satisfy the relations
satisfy the relations  . As a result the configuration integral is simply
. As a result the configuration integral is simply  .
.
 . This specific form allowed evaluation of the
. This specific form allowed evaluation of the 
 , so that
, so that

 . They found, that a second limiting process they called the van der Waals limit,
. They found, that a second limiting process they called the van der Waals limit,  (in which the pair potential becomes both infinitely long range and infinity weak) and performed after the thermodynamic limit, produced the one-dimensional vdW equation (here rendered in molar form)
(in which the pair potential becomes both infinitely long range and infinity weak) and performed after the thermodynamic limit, produced the one-dimensional vdW equation (here rendered in molar form)
 and
and  ,
together with the Gibbs criterion,
,
together with the Gibbs criterion,  (equivalently the
(equivalently the  as shown in Fig. 9, and hence the first criticism of the vdW equation is not as serious as originally thought.
as shown in Fig. 9, and hence the first criticism of the vdW equation is not as serious as originally thought.
 , of dimensions,
, of dimensions,  . For
. For  and
and  this reduces to the specific one-dimensional function considered by Kac, et. al. and for
this reduces to the specific one-dimensional function considered by Kac, et. al. and for  it is an arbitrary function (although subject to specific requirements) in physical three dimensional space. In fact the function
it is an arbitrary function (although subject to specific requirements) in physical three dimensional space. In fact the function  must be bounded, non-negative, and one whose integral
must be bounded, non-negative, and one whose integral

 and hence on
and hence on  , taking the thermodynamic limit (
, taking the thermodynamic limit ( ) to obtain upper and lower bounds on the function
) to obtain upper and lower bounds on the function  , then subsequently taking the van der Waals limit, they found that the two bounds coalesced and thereby produced a unique limit, here written in terms of the free energy per mole and the molar volume,
, then subsequently taking the van der Waals limit, they found that the two bounds coalesced and thereby produced a unique limit, here written in terms of the free energy per mole and the molar volume,

 is the limit function when
is the limit function when  ; also here
; also here  . This result is illustrated in the present context by the solid green curves and black line in Fig. 8, which is the convex envelope of
. This result is illustrated in the present context by the solid green curves and black line in Fig. 8, which is the convex envelope of 
 is the pressure when attractive molecular forces are absent.
is the pressure when attractive molecular forces are absent.
 the pressure that results from direct particle collisions (or more accurately the core repulsive forces), replaces
the pressure that results from direct particle collisions (or more accurately the core repulsive forces), replaces  . This is consistent with the second criticism that can be stated as
. This is consistent with the second criticism that can be stated as  . Consequently the vdW equation cannot be rigorously derived from the configuration integral over the entire range of
. Consequently the vdW equation cannot be rigorously derived from the configuration integral over the entire range of  .
.
 (see above for its definition),
(see above for its definition),
 , together with the number of particles
, together with the number of particles  Substituting the expression for
Substituting the expression for  produces
produces

 in its convergent power series, using the series for
in its convergent power series, using the series for  in each term, and equating powers of
in each term, and equating powers of  produces relations that can be solved for the
produces relations that can be solved for the  in terms of the
in terms of the  . For example
. For example  ,
,  , and
, and  .
.
 , the number density,
, the number density,  , is expressed as the series
, is expressed as the series

 are given in terms of
are given in terms of  , and equating powers of
, and equating powers of  , etc. Finally, using this series in the series for
, etc. Finally, using this series in the series for 

 , and a finite number of terms is an
, and a finite number of terms is an  . The dominant order approximation in this limit is
. The dominant order approximation in this limit is  , which is the ideal gas law. It can be written as an equality using
, which is the ideal gas law. It can be written as an equality using  , which states that the remaining terms approach zero in the limit, or
, which states that the remaining terms approach zero in the limit, or  , which states, more accurately, that they approach zero in proportion to
, which states, more accurately, that they approach zero in proportion to  , and the expression for
, and the expression for 
 and
and  is a dimensionless two particle potential function. For spherically symetric molecules this function can be represented most simply with two parameters,
is a dimensionless two particle potential function. For spherically symetric molecules this function can be represented most simply with two parameters,  , a characteristic molecular diameter, and binding energy respectively as shown in the accompanying plot in which
, a characteristic molecular diameter, and binding energy respectively as shown in the accompanying plot in which  . Also for spherically symetric molecules 5 of the 6 integrals in the expression for
. Also for spherically symetric molecules 5 of the 6 integrals in the expression for 
 is positive for
is positive for  , and negative for
, and negative for  with a minimum of
with a minimum of  at some
at some  . Furthermore
. Furthermore  increases so rapidly that whenever
increases so rapidly that whenever  . In addition in the limit
. In addition in the limit  (
( is a dimensionless coldness, and the quantity
is a dimensionless coldness, and the quantity  is a characteristic molecular temperature) the exponential can be approximated for
is a characteristic molecular temperature) the exponential can be approximated for  can be approximated as
can be approximated as

 . On splitting the interval of integration into 2 parts, one less than and the other greater than
. On splitting the interval of integration into 2 parts, one less than and the other greater than 
 and
and  with
with 
 where
where  be integrable over the range [1,
be integrable over the range [1, ). This result indicates that a dimensionless
). This result indicates that a dimensionless  that is a function of a dimensionless molecular temperature
that is a function of a dimensionless molecular temperature  ; this is an example of the principle of corresponding states on the molecular level. Moreover this is true in general and has been developed extensively both theoretically and experimentally.
; this is an example of the principle of corresponding states on the molecular level. Moreover this is true in general and has been developed extensively both theoretically and experimentally.


 , uniformly convergent for
, uniformly convergent for  , can be written as
, can be written as  , so substituting for
, so substituting for  produces
produces
 Alternatively this is
Alternatively this is
 the vdW equation.
the vdW equation.
 . Consequently the equation produces an accurate approximation in a region defined by
. Consequently the equation produces an accurate approximation in a region defined by  (on a molecular basis
(on a molecular basis  ), which corresponds to a dilute gas. But as the density becomes larger the behavior of the vdW approximation and the 2 term virial expansion differ markedly. Whereas the virial approximation in this instance either increases or decreases continuously, the vdW approximation together with the Maxwell construction expresses physical reality in the form of a phase change, while also indicating the existence of metastable states. This difference in behaviors was pointed out long ago by Korteweg, and Rayleigh (see Rowlinson) in the course of their dispute with
), which corresponds to a dilute gas. But as the density becomes larger the behavior of the vdW approximation and the 2 term virial expansion differ markedly. Whereas the virial approximation in this instance either increases or decreases continuously, the vdW approximation together with the Maxwell construction expresses physical reality in the form of a phase change, while also indicating the existence of metastable states. This difference in behaviors was pointed out long ago by Korteweg, and Rayleigh (see Rowlinson) in the course of their dispute with