| vanillyl-alcohol oxidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Vanillyl-alcohol oxidase homooctamer, Penicillium simplicissimum Vanillyl-alcohol oxidase homooctamer, Penicillium simplicissimum | |||||||||
| Identifiers | |||||||||
| EC no. | 1.1.3.38 | ||||||||
| CAS no. | 143929-24-2 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
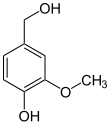
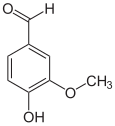
In enzymology, a vanillyl-alcohol oxidase (EC 1.1.3.38) is an enzyme that catalyzes the chemical reaction
Thus, the two substrates of this enzyme are vanillyl alcohol and O2, whereas its two products are vanillin and H2O2.
This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-OH group of donor with oxygen as acceptor. The systematic name of this enzyme class is vanillyl alcohol:oxygen oxidoreductase. This enzyme is also called 4-hydroxy-2-methoxybenzyl alcohol oxidase. This enzyme participates in 2,4-dichlorobenzoate degradation. It employs one cofactor, FAD.
Structural studies
As of late 2007, 3 structures have been solved for this class of enzymes, with PDB accession codes 1DZN, 1E0Y, and 1E8G.
References
- de Jong E, van Berkel WJ, van der Zwan RP, de Bont JA (1992). "Purification and characterization of vanillyl-alcohol oxidase from Penicillium simplicissimum. A novel aromatic alcohol oxidase containing covalently bound FAD". Eur. J. Biochem. 208 (3): 651–657. doi:10.1111/j.1432-1033.1992.tb17231.x. PMID 1396672.
- Fraaije MW, Veeger C, van Berkel WJ (1995). "Substrate specificity of flavin-dependent vanillyl-alcohol oxidase from Penicillium simplicissimum. Evidence for the production of 4-hydroxycinnamyl alcohols from 4-allylphenols" (PDF). Eur. J. Biochem. 234 (1): 271–277. doi:10.1111/j.1432-1033.1995.271_c.x. PMID 8529652.
| Enzymes | |
|---|---|
| Activity | |
| Regulation | |
| Classification | |
| Kinetics | |
| Types |
|
This EC 1.1 enzyme-related article is a stub. You can help Misplaced Pages by expanding it. |