| |
| |
| Names | |
|---|---|
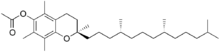
| Preferred IUPAC name (2R)-2,5,7,8-Tetramethyl-2--3,4-dihydro-2H-1-benzopyran-6-yl acetate | |
| Other names
α-Tocopherol acetate Vitamin E acetate | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.369 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C31H52O3 |
| Molar mass | 472.743 g/mol |
| Appearance | pale yellow, viscous liquid |
| Melting point | –27.5 °C |
| Boiling point | 240 °C decays without boiling |
| Solubility in water | insoluble |
| Solubility | soluble in acetone, chloroform, diethyl ether; poorly soluble in ethanol |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
α-Tocopheryl acetate (alpha-tocopherol acetate), also known as vitamin E acetate, is a form of vitamin E with D-Alpha Tocopheryl Acetate as the natural form and DL-Alpha Tocopheryl Acetate as the synthetic form. DL-indicates the synthetic form where as D- indicates the natural form. It is the ester of acetic acid and α-tocopherol.
The U.S. Centers for Disease Control and Prevention says that vitamin E acetate is a very strong culprit of concern in the 2019 outbreak of vaping-associated pulmonary injury (VAPI), but there is not yet sufficient evidence to rule out contributions from other chemicals. Vaporization of this ester produces toxic pyrolysis products.
Use in cosmetics
α-Tocopheryl acetate is often used in dermatological products such as skin creams. It is not oxidized and can penetrate through the skin to the living cells, where about 5% is converted to free tocopherol. Claims are made for beneficial antioxidant effects. α-Tocopheryl acetate is used as an alternative to tocopherol itself because the phenolic hydroxyl group is blocked, providing a less acidic product with a longer shelf life. It is believed that the acetate is slowly hydrolyzed after it is absorbed into the skin, regenerating tocopherol and providing protection against the sun's ultraviolet rays. Tocopheryl acetate was first synthesized in 1963 by workers at Hoffmann-La Roche.
Although there is widespread use of tocopheryl acetate as a topical medication, with claims for improved wound healing and reduced scar tissue, reviews have repeatedly concluded that there is insufficient evidence to support these claims. There are reports of vitamin E-induced allergic contact dermatitis from use of vitamin E derivatives such as tocopheryl linoleate and tocopherol acetate in skin care products. Incidence is low despite widespread use.
Misuse
Ingredient in vape liquids
See also: 2019–20 vaping lung illness outbreak, Vaping-associated pulmonary injury, and Lacing (drugs) § CannabisOn September 5, 2019, the United States Food and Drug Administration (US FDA) announced that 10 out of 18, or 56% of the samples of vape liquids sent in by states, linked to the recent vaping-related lung disease outbreak in the United States, tested positive for vitamin E acetate which had been used as a thickening agent by illicit THC vape cartridge manufacturers. On November 8, 2019, the Centers for Disease Control and Prevention (CDC) identified vitamin E acetate as a very strong culprit of concern in the vaping-related illnesses, but has not ruled out other chemicals or toxicants as possible causes. The CDC's findings were based on fluid samples from the lungs of 29 patients with vaping-associated pulmonary injury, which provided direct evidence of vitamin E acetate at the primary site of injury in all the 29 lung fluid samples tested. Research suggests when vitamin E acetate is inhaled, it may interfere with normal lung functioning. A 2020 study found that vaporizing vitamin E acetate produced carcinogenic alkenes and benzene, but also exceptionally toxic ketene gas, which may be a contributing factor to the pulmonary injuries.
Chemistry
At room temperature, α-tocopheryl acetate is a fat-soluble liquid. It has 3 chiral centers and thus 8 stereoisomers. It is made by esterifying α-tocopherol with acetic acid. 2R,4R,8R-isomer, also known as RRR-α-tocopheryl acetate, is the most common isomer used for various purposes. This is because α-tocopherol occurs in nature primarily as RRR-α-tocopherol.
α-Tocopherol acetate does not boil at atmospheric pressure and begins to degrade at 240 °C. It can be vacuum distilled: it boils at 184 °C at 0.01 mmHg, at 194 °C (0.025 mmHg) and at 224 °C (0.3 mmHg). In practice, it is not degraded notably by air, visible light or UV-radiation. It has a refractive index of 1.4950–1.4972 at 20 °C.
α-Tocopherol acetate is hydrolyzed to α-tocopherol and acetic acid under suitable conditions or when ingested by people.
References
- ^ The Merck index (12th ed.). Merck. 1996. p. 1580. ISBN 9780911910124.
- ^ "Safety assessment of the substance α-tocopherol acetate for use in food contact materials". EFSA Journal. 14 (3): 4412. 2016. doi:10.2903/j.efsa.2016.4412.
- ^ "Transcript of CDC Telebriefing: Update on Lung Injury Associated with E-cigarette Use, or Vaping". Centers for Disease Control and Prevention. November 8, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- Feldman R, Meiman J, Stanton M, Gummin DD (June 2020). "Culprit or correlate? An application of the Bradford Hill criteria to Vitamin E acetate". Archives of Toxicology. 94 (6): 2249–2254. doi:10.1007/s00204-020-02770-x. ISSN 1432-0738. PMID 32451600. S2CID 218878143.
- ^ "Outbreak of Lung Injury Associated with E-Cigarette Use, or Vaping". Centers for Disease Control and Prevention. November 8, 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Wu D, O'Shea DF (March 24, 2020). "Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate". Proceedings of the National Academy of Sciences of the United States of America. 117 (12): 6349–6355. Bibcode:2020PNAS..117.6349W. doi:10.1073/pnas.1920925117. PMC 7104367. PMID 32156732.
- Linus Pauling Institute Research Report: All About E at the Wayback Machine (archived February 23, 2015)
- Beijersbergen van Henegouwen G, Junginger H, de Vries H (1995). "Hydrolysis of RRR-alpha-tocopheryl acetate (vitamin E acetate) in the skin and its UV protecting activity (an in vivo study with the rat)". J Photochem Photobiol B. 29 (1): 45–51. doi:10.1016/1011-1344(95)90251-1. PMID 7472802.
- Mayer H, Schudel P, Rüegg R, Isler O (1963). "Über die Chemie des Vitamins E. 3. Mitteilung. Die Totalsynthese von (2R, 4′R, 8′R)- und (2S, 4′R, 8′R)-α-Tocopherol". Helvetica Chimica Acta. 46 (2): 650–671. doi:10.1002/hlca.19630460225. ISSN 0018-019X.
- Panin G, Strumia R, Ursini F (2004). "Topical alpha-tocopherol acetate in the bulk phase: eight years of experience in skin treatment" (PDF). Ann. N. Y. Acad. Sci. 1031 (1): 443–447. Bibcode:2004NYASA1031..443P. doi:10.1196/annals.1331.069. PMID 15753192. S2CID 45771699.
- Sidgwick GP, McGeorge D, Bayat A (2015). "A comprehensive evidence-based review on the role of topicals and dressings in the management of skin scarring". Arch. Dermatol. Res. 307 (6): 461–477. doi:10.1007/s00403-015-1572-0. PMC 4506744. PMID 26044054.
- Tanaydin V, Conings J, Malyar M, van der Hulst R, van der Lei B (2016). "The Role of Topical Vitamin E in Scar Management: A Systematic Review". Aesthet Surg J. 36 (8): 959–965. doi:10.1093/asj/sjw046. PMID 26977069.
- Kosari P, Alikhan A, Sockolov M, Feldman SR (2010). "Vitamin E and allergic contact dermatitis". Dermatitis. 21 (3): 148–153. doi:10.2310/6620.2010.09083. PMID 20487657. S2CID 38212099.
- Sun L (September 6, 2019). "Contaminant found in marijuana vaping products linked to deadly lung illnesses, tests show". Washington Post. Retrieved September 9, 2019.
- "Three Companies Subpoenaed in Weed Vape Illness Investigation". Rolling Stone. September 10, 2019.