
| |
| Names | |
|---|---|
| IUPAC name (22R)-4β,27-Dihydroxy-5,6β:22,26-diepoxy-5β-ergosta-2,24-diene-1,26-dione | |
| Systematic IUPAC name (4S,4aR,5aR,6aS,6bS,9R,9aS,11aS,11bR)-4-Hydroxy-9-{(1S)-1-ethyl}-9a,11b-dimethyl-5a,6,6a,6b,7,8,9,9a,10,11,11a,11b-dodecahydrocyclopentaphenanthrooxiren-1(4H)-one | |
| Other names Withaferine A | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C28H38O6 |
| Molar mass | 470.606 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Withaferin A is a steroidal lactone, derived from Acnistus arborescens, Withania somnifera and other members of family Solanaceae. It is the first member of the withanolide class of ergostane type product to be discovered.
Structure
Withanolides are a group of naturally occurring C28- steroidal lactones. They contain four cycloalkane ring structures, three cyclohexane rings and one cyclopentane ring. Withaferin A is highly reactive because of the ketone-containing unsaturated A ring, the epoxide in the B ring, and the unsaturated lactone ring. The double bond in ring A and the epoxide ring are mainly responsible for the cytotoxicity. The 22nd and 26th carbons of the ergostane skeleton in withaferin A and related steroidal compounds are oxidized to form a six-membered delta lactone unit. NMR spectral analysis identifies C3 in the unsaturated A ring as the main nucleophilic target site for ethyl mercaptan, thiophenol and L-cysteine ethyl ester in vitro. A library of 2, 3-dihydro-3β-substituted derivatives are synthesized by regio/stereoselective Michael addition to ring A.
Regulation
Transcription factor NF-κB in vitro
NF-κB is a transcription factor that regulates many genes involved in cell survival, growth, immune response and angiogenesis. Withaferin A inhibits NF-κB at a very low concentration by targeting the ubiquitin-mediated proteasome pathway (UPP) in endothelial cells. In vitro experiments demonstrated that withaferin A inhibits other transcription factors including Ap1 and Sp1.
Biosynthesis

In the Withania somnifera plant, the withaferin A is present in the leaves. Withanolides are terpenoids, which are synthesized in plants using isoprenoids as precursors. Isoprenoids can be synthesized through mevalonate or 1-deoxy-D-xylulose 5-phosphate pathways. Isoprenogenesis significantly governs withanolide synthesis.
Isoprenoids form squalene, which then goes through a variety of intermediate steps to form 24-methylenecholesterol - the sterol precursor of the withanolides.
The biosynthesis of withaferin A uses enzymes such as squalene epoxidase (SQE), cycloartenol synthase (CAS), sterol methyl transferase (SMT), obtusifoliol-14 –demethylase (ODM).
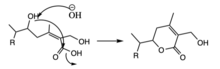
To produce withaferin A from 24-methylene cholesterol, the molecule undergoes several functional changes including formation of a ketone, epoxide, 2 hydroxyl groups, and lactone ring.
See also
References
- Kupchan, S. M.; Anderson, W. K.; Bollinger, P.; Doskotch, R. W.; Smith, R. M.; Renauld, J. A.; Schnoes, H. K.; Burlingame, A. L.; Smith, D. H. (1969-12-01). "Tumor inhibitors. XXXIX. Active principles of Acnistus arborescens. Isolation and structural and spectral studies of withaferin A and withacnistin". The Journal of Organic Chemistry. 34 (12): 3858–3866. doi:10.1021/jo01264a027. PMID 5357526.
- ^ Mohan, R; Hammers, HJ; Bargagna-Mohan, P; Zhan, XH; Herbstritt, CJ; Ruiz, A; Zhang, L; Hanson, AD; et al. (2004). "Withaferin A is a potent inhibitor of angiogenesis". Angiogenesis. 7 (2): 115–122. doi:10.1007/s10456-004-1026-3. PMID 15516832. S2CID 8095820.
- ^ Vanden Berghe, Wim; Sabbe, Linde; Kaileh, Mary; Haegeman, Guy; Heyninck, Karen (2012-11-15). "Molecular insight in the multifunctional activities of Withaferin A". Biochemical Pharmacology. 84 (10): 1282–1291. doi:10.1016/j.bcp.2012.08.027. PMID 22981382.
- Braun, Lesley; Cohen, Marc (2015-03-30). Herbs and Natural Supplements, Volume 2: An Evidence-Based Guide. Elsevier Health Sciences. ISBN 978-0-7295-8173-8.
- Prasanna Kumar, S; Shilpa, P; Salimath Bharati, P (2009). "Withaferin A suppresses the expression of vascular endothelial growth factor in Ehrlich ascites tumor cells via Sp1 transcription factor". Current Trends in Biotechnology and Pharmacy. 3 (2): 138–148. ISSN 0973-8916.
- Chaurasiya, Narayan D.; Sangwan, Neelam S.; Sabir, Farzana; Misra, Laxminarain; Sangwan, Rajender S. (2012). "Withanolide biosynthesis recruits both mevalonate and DOXP pathways of isoprenogenesis in Ashwagandha Withania somnifera L. (Dunal)". Plant Cell Reports. 31 (10): 1889–1897. doi:10.1007/s00299-012-1302-4. PMID 22733207. S2CID 253817403.
- Lockley, William J.S.; Rees, Huw H.; Goodwin, Trevor W. (1976). "Biosynthesis of steroidal withanolides in Withania somnifera". Phytochemistry. 15 (6): 937–939. Bibcode:1976PChem..15..937L. doi:10.1016/S0031-9422(00)84374-5.
- Pandey, Shiv S.; Singh, Sucheta; Pandey, Harshita; Srivastava, Madhumita; Ray, Tania; Soni, Sumit; Pandey, Alok; Shanker, Karuna; Babu, C. S. Vivek; Banerjee, Suchitra; Gupta, M. M.; Kalra, Alok (2018). "Endophytes of Withania somnifera modulate in planta content and the site of withanolide biosynthesis". Scientific Reports. 8 (1): 5450. Bibcode:2018NatSR...8.5450P. doi:10.1038/s41598-018-23716-5. PMC 5882813. PMID 29615668.
- Bharitkar, Yogesh P.; Kanhar, Satish; Suneel, Neradibilli; Mondal, Susanta Kumar; Hazra, Abhijit; Mondal, Nirup B. (2015). "Chemistry of withaferin-A: Chemo, regio, and stereoselective synthesis of novel spiro-pyrrolizidino-oxindole adducts of withaferin-A via one-pot three-component [3+2] azomethine ylide cycloaddition and their cytotoxicity evaluation". Molecular Diversity. 19 (2): 251–261. doi:10.1007/s11030-015-9574-6. PMID 25749788. S2CID 254831740.