| |

| |
| |
| Names | |
|---|---|
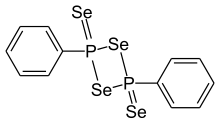
| Preferred IUPAC name 2,4-Diphenyl-1,3,2λ,4λ-diselenadiphosphetane-2,4-diselone | |
| Other names Woollins' Reagent | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.155.582 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C12H10P2Se4 |
| Molar mass | 532.044 g·mol |
| Appearance | red powder |
| Melting point | 192 to 204 °C (378 to 399 °F; 465 to 477 K) |
| Solubility in water | soluble in toluene at elevated temperatures |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H301, H331, H373, H410 |
| Related compounds | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Woollins' reagent is an organic compound containing phosphorus and selenium. Analogous to Lawesson's reagent, it is used mainly as a selenation reagent. It is named after John Derek Woollins.
Preparation
Woollins' reagent is commercially available. It can also be conveniently prepared in the laboratory by heating a mixture of dichlorophenylphosphine and sodium selenide (Na2Se), (itself prepared from reacting elementary selenium with sodium in liquid ammonia). An alternative synthesis is the reaction of the pentamer (PPh)5 (pentaphenylcyclopentaphosphine) with elemental selenium.
Applications
The main use of Woollins' reagent is the selenation of carbonyl compounds. For instance, Woollins' reagent will convert a carbonyl into a selenocarbonyl. Additionally, Woollins' reagent has been used to selenonate carboxylic acids, alkenes, alkynes, and nitriles.
References
- The Merck Index. An Encyclopaedia of Chemicals, Drugs and Biologicals. 14. Auflage, 2006, S. 1731, ISBN 978-0-911910-00-1
- Ian P. Gray, Pravat Bhattacharyya, Alexandra Slawin and J. Derek Woollins (2005). "A New Synthesis of (PhPSe2)2 (Woollins Reagent) and Its Use in the Synthesis of Novel P-Se Heterocycles". Chem. Eur. J. 11 (21): 6221–7. doi:10.1002/chem.200500291. PMID 16075451.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - M. J. Pilkington, Alexandra M. Z. Slawin and J. Derek Woollins (1990). "The preparation and characterization of binary phosphorus-selenium rings". Heteroatom Chemistry. 1 (5): 351–355. doi:10.1002/hc.520010502.
- Pravat Bhattacharyya & J. Derek Woollins (2001). "Selenocarbonyl synthesis using Woollins reagent". Tetrahedron Lett. 42 (34): 5949. doi:10.1016/S0040-4039(01)01113-3. hdl:10023/1776.
- Guoxiong Hua & J. Derek Woollins (2009). "Formation and Reactivity of Phosphorus-Selenium Rings". Angew. Chem. Int. Ed. 48 (8): 1368–1377. doi:10.1002/anie.200800572. PMID 19053094.