| YopR_core | |||||||||
|---|---|---|---|---|---|---|---|---|---|
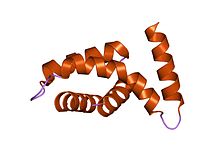 crystal structure of the core domain of yersinia pestis virulence factor yopr crystal structure of the core domain of yersinia pestis virulence factor yopr | |||||||||
| Identifiers | |||||||||
| Symbol | YopR_core | ||||||||
| Pfam | PF09025 | ||||||||
| InterPro | IPR013349 | ||||||||
| |||||||||
In molecular biology, YopR is a protein domain commonly found in gram negative bacteria, in particular Yersinia and is a core domain. Proteins in this entry are type III secretion system effectors. They are named differently in different species and in Yersinia has been designated YopR (Yersinia outer protein R) which is encoded by the YscH (Yersinia secretion H) gene. This Yop protein is unusual in that it is released to the extracellular environment rather than injected directly into the target cell as are most Yop proteins. A hallmark of Yersinia type III machines is the presence of needles extending from the bacterial surface. Needles perform two functions, firstly, as a channel to export effectors into the immune cells and secondly as a sensor.
Function
In summary, YopR may play a role in the regulation of type III secretion. It is important to note, however, that the precise function of the YopR domain remains to be elucidated but YopR is thought to be involved in with YscF which develops needles. There are two different hypotheses for this. The first explanation is that YopR is possibly controlling the secretion of YscF, which impacts the assembly of type III machines. Alternatively, it is thought that YopR directly polymerizes YscF; this explanation is thought to be less likely since YopR does not associated with purified needles.
Structure
YopR, is composed of five alpha-helices, four of which are arranged in an antiparallel bundle.
Secretion systems
There have been four secretion systems described in animal enteropathogens, such as Salmonella and Yersinia, with further sequence similarities in plant pathogens like Ralstonia and Erwinia. This is useful for further work with the YopR protein domain as comparative sequence and structure homology can be made. The type III secretion system is of great interest, as the YopR protein domain plays an important role. The type III secretion system transports virulence factors from the pathogen directly into the host cell when the bacterium is in close contact with the host.
References
- ^ Schubot FD, Cherry S, Austin BP, Tropea JE, Waugh DS (2005). "Crystal structure of the protease-resistant core domain of Yersinia pestis virulence factor YopR". Protein Sci. 14 (6): 1679–83. doi:10.1110/ps.051446405. PMC 2253397. PMID 15930010.
- Hueck CJ (June 1998). "Type III protein secretion systems in bacterial pathogens of animals and plants". Microbiol. Mol. Biol. Rev. 62 (2): 379–433. doi:10.1128/MMBR.62.2.379-433.1998. PMC 98920. PMID 9618447.