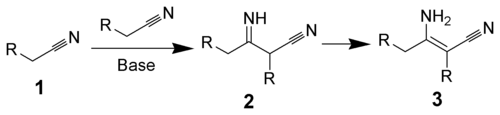
The Thorpe reaction is a chemical reaction described as a self-condensation of aliphatic nitriles catalyzed by base to form enamines. The reaction was discovered by Jocelyn Field Thorpe.
Thorpe–Ziegler reaction
The Thorpe–Ziegler reaction (named after Jocelyn Field Thorpe and Karl Ziegler), or Ziegler method, is the intramolecular modification with a dinitrile as a reactant and a cyclic ketone as the final reaction product after acidic hydrolysis. The reaction is conceptually related to the Dieckmann condensation.

References
- Baron, Harold; Remfry, Frederick George Percy; Thorpe, Jocelyn Field (1904). "CLXXV.—The formation and reactions of imino-compounds. Part I. Condensation of ethyl cyanoacetate with its sodium derivative". Journal of the Chemical Society, Transactions. 85: 1726–1761. doi:10.1039/CT9048501726. ISSN 0368-1645.
- Ziegler, K.; Eberle, H.; Ohlinger, H. (1933). "Über vielgliedrige Ringsysteme. I. Die präparativ ergiebige Synthese der Polymethylenketone mit mehr als 6 Ringgliedern". Justus Liebig's Annalen der Chemie (in German). 504 (1): 94–130. doi:10.1002/jlac.19335040109. ISSN 0075-4617.
- ^ Schaefer, John P.; Bloomfield, Jordan J. (2011). "The Dieckmann Condensation (Including the Thorpe-Ziegler Condensation)". Organic Reactions. pp. 1–203. doi:10.1002/0471264180.or015.01. ISBN 978-0471264187.
External links
- Thorpe-Ziegler reaction: 4-Phosphorinanone, 1-phenyl- Organic Syntheses, Coll. Vol. 6, p. 932 (1988); Vol. 53, p. 98 (1973) Link