| Revision as of 18:40, 17 October 2007 edit208.68.154.85 (talk) →Retirement← Previous edit | Latest revision as of 19:49, 9 December 2024 edit undoMichaelsexton2003 (talk | contribs)Extended confirmed users1,669 edits →(Top): Birthplace and deathplace as per Template:Infobox person | ||
| Line 1: | Line 1: | ||
| {{ |
{{Short description|Italian physicist and chemist (1745–1827)}} | ||
| {{For|the concept car|Toyota Alessandro Volta}} | |||
| {{Infobox_Scientist | |||
| {{pp-pc1|small=yes}} | |||
| | name = Alessandro Volta | |||
| <!--Galvani and "Early battery" sections need more citations--> | |||
| | image = Alessandro Volta.jpeg|225px | |||
| {{use British English|date=February 2015}} | |||
| | image_width = 225px | |||
| {{Use dmy dates|date=March 2023}} | |||
| | caption = Alessandro Giuseppe Antonio Anastasio Volta (1745-1827) | |||
| {{Infobox scientist | |||
| | birth_date = {{birth date|1745|2|18|mf=y}} | |||
| | honorific_prefix = ] | |||
| | birth_place = ] ], ], ] | |||
| | honorific_suffix = {{post-nominals|country=GBR|size=100%|ForMemRS}} | |||
| | death_date = {{death date and age|1827|3|5|1745|2|18|mf=y}} | |||
| | image = Alessandro Volta.jpeg | |||
| | death_place = ] ], ], ] | |||
| | birth_name = Alessandro Giuseppe Antonio Anastasio Volta | |||
| | residence = ] ] | |||
| | |
| birth_date = {{birth date|df=y|1745|2|18}} | ||
| | |
| birth_place = ], ] | ||
| | death_date = {{death date and age|df=y|1827|3|5|1745|2|18}} | |||
| | work_institutions = | |||
| | death_place = Como, ], ] | |||
| | alma_mater = | |||
| | known_for = ]<br>] | |||
| | doctoral_advisor = | |||
| | spouse = {{marriage|Teresa Peregrini|1794}} | |||
| | doctoral_students = | |||
| | children = 3 | |||
| | known_for = Development of the ] | |||
| | awards = {{ubl|] (1791)|] (1794)|] (1805)|] (1806)}} | |||
| | author_abbrev_bot = | |||
| | fields = ]<br>] | |||
| | author_abbrev_zoo = | |||
| | work_institutions = ] | |||
| | prizes = | |||
| | religion = | |||
| | footnotes = | |||
| }} | }} | ||
| {{electromagnetism|Scientists}} | |||
| '''Alessandro Giuseppe Antonio Anastasio Volta''' ({{IPAc-en|UK|ˈ|v|ɒ|l|t|ə}}, {{IPAc-en|US|ˈ|v|oʊ|l|t|ə}}; {{IPA|it|alesˈsandro ˈvɔlta|lang}}; 18 February 1745 – 5 March 1827) was an Italian ] and ] who was a pioneer of ] and ],<ref name="pancaldi" /><ref name="berzolari" /><ref name="edison" /> and is credited as the inventor of the ] and the discoverer of ]. He invented the ] in 1799, and reported the results of his experiments in a two-part letter to the president of the ],<ref name="ieeeghn" /><ref name="royal" /> which was published in 1800.<ref name="volta-1800-pht">{{cite journal|title=On the Electricity excited by the mere Contact of conducting Substances of different kinds.|journal=]|date=20 March 1800|first=Alessandro|last=Volta|authorlink=Alessandro Volta|pages=403–431|editor-first=Joseph|editor-last=Banks|editor-link=Joseph Banks|language=fr|doi=10.1098/rstl.1800.0018|url=https://zenodo.org/record/1432304|volume=90 }}</ref> With this invention, Volta proved that electricity could be generated chemically and debunked the prevalent theory that electricity was generated solely by living beings. Volta's invention sparked a great amount of scientific excitement and led others to conduct similar experiments, which eventually led to the development of the field of ].<ref name="royal" /> | |||
| '''] Alessandro Giuseppe Antonio Anastasio Volta''' (], ] - ], ]) was an ] ] known especially for the development of the ] in 1800. | |||
| Volta drew admiration from ] for his invention, and was invited to the ] to demonstrate his invention to the members of the institute. Throughout his life, Volta enjoyed a certain amount of closeness with the emperor who conferred upon him numerous honours.<ref name="munro" /> Volta held the chair of experimental physics at the ] for nearly 40 years and was widely idolised by his students.<ref name="munro" /> Despite his professional success, Volta was inclined towards domestic life and this was more apparent in his later years when he tended to live secluded from public life and more for the sake of his family. He died in 1827 from a series of illnesses which began in 1823.<ref name="munro" /> The ] unit of ] is named the ] in his honour. | |||
| == Career == | |||
| ==Early life and marriage == | |||
| In 1774, he became professor of physics in the Como high school. His passion had always been the study of electricity, and while still a young student he had even written a poem in ] on this fascinating new discovery. His first scientific paper he titled ''De vi attractiva ignis electrici ac phaenomenis inde pendentibus''. | |||
| Volta was born in ], a town in northern ], on 18 February 1745. His father, Filippo Volta, was of noble lineage. His mother, Donna Maddalena, came from the family of the Inzaghis.<ref name="lifeworks" /> In 1794, Volta married an aristocratic lady also from Como, Teresa Peregrini, with whom he raised three sons: Zanino, Flaminio, and Luigi. | |||
| ==Career== | |||
| ] | |||
| In 1774, he became a professor of physics at the Royal School in Como. A year later, he improved and popularised the ], a device that produced ]. His promotion of it was so extensive that he is often credited with its invention, even though a machine operating on the same principle was described in 1762 by the Swedish experimenter ].<ref name="pancaldi" /><ref name="wilcke" /> In 1777, he travelled through Switzerland, where he befriended ]. | |||
| ==Inventions and discoveries== | |||
| In the years between 1776 and 1778, Volta studied the ] of gases. He researched and discovered ] after reading a paper by ] of the United States on "flammable air". In November 1776, he found methane in the marshes of ] on ],<ref name="marsh" /> and by 1778 he managed to isolate it.<ref name="methane" /> He devised experiments such as the ] of methane by an electric ] in a closed vessel. | |||
| In 1775 he invented the ], a device that produced a static electric charge. In 1776-] he studied the ] of ]es, discovered ], and devised experiments such as the ] of gases by an electric ] in a closed vessel. Volta also studied what we now call capacitance, developing separate means to study both electrical potential V and charge Q, and to discovering that for a given object they are proportional. This may be called Volta's Law of ], and likely for this work the unit of electrical potential has been named the ]. | |||
| In 1779 he became professor of experimental physics at the University of ], a chair he occupied for almost 40 years. In 1794, Volta married the daughter of Count Ludovico Peregrini, Teresa, with whom he raised three sons. Each of his three sons went on to improve on the Voltaic Pile in ways that all ended in strait ends. | |||
| Volta also studied what we now call electrical ], developing separate means to study both electrical potential difference (''V'') and charge (''Q''), and discovering that for a given object, they are proportional.<ref name="williams" /> This is called Volta's Law of Capacitance, and for this work, the unit of electrical potential has been named the volt.<ref name="williams" /> | |||
| Around 1791 he began to study the "animal electricity" noted by ] when two different metals were connected in series with the frog's leg and to one another. He realized that the frog's leg served as both a conductor of electricity (we would now call it an ]) and as a detector of electricity. He replaced the frog's leg by brine-soaked paper, and detected the flow of electricity by other means familiar to him from his previous studies of electricity. In this way he discovered the ], and the law that the ] (emf) of a ], consisting of a pair of metal ]s separated by electrolyte, is the difference of their two electrode potentials. That is, if the electrodes have emfs <math>\mathcal{E}_{1,2}</math>, then the net emf is <math>\mathcal{E}_{2}-\mathcal{E}_{1}</math>. (Thus, two identical electrodes and a common electrolyte give zero net emf.) This may be called Volta's Law of the electrochemical series. | |||
| In 1779, he became a professor of experimental physics at the ], a chair that he occupied for almost 40 years.<ref name="munro" /> Volta's lectures were so crowded with students that the subsequent emperor ] ordered the construction (based on a project by ]) of a new "physical theatre", today the "Aula Volta".<ref name="Aula Volta">{{cite web|url=https://alessandrovolta.it/luoghi-voltiani/pavia/aula-volta/|title=Aula Volta|work=Luoghi Voltiani|access-date=21 August 2022}}</ref> Furthermore, the emperor granted Volta substantial funding to equip the physics cabinet with instruments, purchased by Volta in England and France. At the ] of the University of Pavia there are 150 of them, used by Alessandro Volta.<ref name="Sala Volta">{{cite web|url=http://musei.unipv.eu/msu/il-museo/sale/sala-volta/|title=Sala Volta|work=Musei Unipv|access-date=21 August 2022}}</ref><ref>{{cite web|url=https://alessandrovolta.it/luoghi-voltiani/pavia/gabinetto-fisico/|title=Gabinetto Fisico|work=Luoghi Voltiani|access-date=21 August 2022}}</ref> | |||
| In 1800, as the result of a professional disagreement over the galvanic response advocated by ], he invented the ], an early ], which produced a steady electric current. Volta had determined that the most effective pair of dissimilar metals to produce electricity was ] and ]. Initially he experimented with individual cells in series, each cell being a wine goblet filled with ] into which the two dissimilar electrodes were dipped. The electric pile replaced the goblets with cardboard soaked in brine. (The number of cells, and thus the voltage it could produce, was limited by the pressure, exerted by the upper cells, that would squeeze all of the brine out of the cardboard of the bottom cell.) | |||
| == Volta and Galvani == | |||
| In announcing his discovery of the pile, Volta paid tribute to the influences of ], ] and ].<ref>*{{ cite journal | author=Elliott, P. | title=Abraham Bennet F.R.S. (1749-1799): a provincial electrician in eighteenth-century England | journal=Notes and Records of the Royal Society of London | volume=53(1) | pages=59-78 | year=1999 | url=http://www.journals.royalsoc.ac.uk/content/klgdd0umcmvjqnpr/fulltext.pdf |format=PDF}} (</ref> | |||
| ] museum, Como]] | |||
| ], an Italian physicist, discovered something he named "animal electricity" when two different metals were connected in series with a frog's leg and to one another. Volta realised that the frog's leg served as both a conductor of electricity (what we would now call an ]) and as a detector of electricity. He also understood that the frog's legs were irrelevant to the ], which was caused by the two differing metals.<ref>{{cite book |last=Price |first=Derek deSolla |title=On the Brink of Tomorrow: Frontiers of Science |date=1982 |publisher=National Geographic Society |location=Washington D.C. |pages=16–17}}</ref> He replaced the frog's leg with brine-soaked paper and detected the flow of electricity by other means familiar to him from his previous studies. In this way, he discovered the ], and the law that the ] (emf) of a ], consisting of a pair of metal ]s separated by electrolyte, is the difference between their two electrode potentials (thus, two identical electrodes and a common electrolyte give zero net emf). This may be called Volta's Law of the electrochemical series. | |||
| In 1800, as the result of a professional disagreement over the galvanic response advocated by Galvani, Volta invented the ], an early ], which produced a steady electric current.<ref name="routledge" /> Volta had determined that the most effective pair of dissimilar metals to produce electricity was ] and ]. Initially, he experimented with individual cells in series, each cell being a wine goblet filled with ] into which the two dissimilar electrodes were dipped. The voltaic pile replaced the goblets with cardboard soaked in brine. | |||
| ==The Voltaic battery== | |||
| The battery made by Volta is credited to have been the first cell. It consists of two electrodes: one made of ], the other of ]. The ] is ]. The electrolyte exists in the form 2H<sup>+</sup> and SO<sub>4</sub> <sup>2-</sup>. The zinc, which is higher than both copper and hydrogen in the electrochemical series, reacts with the negatively charged sulphate. ( SO<sub>4</sub> ) The positively charged hydrogen bubbles start depositing around the copper and take away some of its ]s. This makes the zinc rod the negative electrode and the copper rod the positive electrode. | |||
| == Early battery == | |||
| We now have 2 terminals, and the current will flow if we connect them. The reactions in this cell are as follows: | |||
| ]]] | |||
| In announcing his discovery of the voltaic pile, Volta paid tribute to the influences of ], ], and ].<ref name="elliott" /> | |||
| The battery made by Volta is credited as one of the first electrochemical cells. It consists of two electrodes: one made of ], the other of ]. The ] is either ] mixed with water or a form of saltwater ]. The electrolyte exists in the form {{chem2|2 H+}} and {{chem2|SO4(2−)}}. Zinc metal, which is higher in the ] than both copper and hydrogen, is oxidized to zinc cations (Zn<sup>2+</sup>) and creates electrons that move to the copper electrode. The positively charged hydrogen ions (]s) capture ]s from the copper electrode, forming bubbles of hydrogen gas, H<sub>2</sub>. This makes the zinc rod the negative electrode and the copper rod the positive electrode. Thus, there are two terminals, and an electric current will flow if they are connected. The ]s in this voltaic cell are as follows: | |||
| {| | |||
| | The zinc | |||
| | Zn -> Zn<sup>2+</sup> + 2e<sup>-</sup> | |||
| |- | |||
| | The copper | |||
| | Cu -> Cu<sup>2+</sup> + 2e<sup>-</sup> | |||
| |- | |||
| | The sulphuric acid | |||
| | H<sub>2</sub>SO<sub>4</sub> -> H<sub>2</sub> + SO<sub>4</sub> | |||
| |} | |||
| :Zinc: | |||
| However, this cell also has some disadvantages. It is unsafe to handle, as sulphuric acid, even if dilute, is dangerous. Also, the potential difference in the terminals finishes after some time. So it is not durable, and therefore, not a suitable choice. | |||
| ::{{chem2|Zn → Zn(2+) + 2e-}} | |||
| :Sulfuric acid: | |||
| ==Honours== | |||
| ::{{chem2|2H+ + 2e- -> H2}} | |||
| In honour of his work in the field of ], ] made him a ] in 1810; in 1815 the ] named him a professor of ] at ]. | |||
| Copper metal does not react, but rather it functions as a ] for the hydrogen-gas formation and an electrode for the electric current. The sulfate anion ({{chem2|SO4(2-)}}) does not undergo any chemical reaction either, but migrates to the zinc anode to compensate for the charge of the zinc cations formed there. However, this cell also has some disadvantages. It is unsafe to handle, since sulfuric acid, even if diluted, can be hazardous. Also, the power of the cell diminishes over time because the hydrogen gas is not released. Instead, it accumulates on the surface of the copper electrode and forms a barrier between the metal and the electrolyte solution. | |||
| Before 1796, ] was ruled by Austria. From 1796 to 1815, Lombardy came under Napoleon's rule. After 1815, Lombardy was once again under Austrian rule. Thus Volta was once a subject of the Emperor of Austria, later a subject of Napoleon and then later a subject of the Emperor of Austria again.<ref>Giuliano Pancaldi, ''"Volta: Science and culture in the age of enlightenment"'', Princeton University Press, 2003.</ref> | |||
| ] | |||
| He was a long-time correspondent of the ] and was made a fellow (]). He received the Society's 1794 ]. He published his invention of the ] battery in 1800 in the ''Philosophical Transactions of the Royal Society''. He was in correspondence with scientists in Austria, which ruled Lombardy in his day, and inm France: in fact his 1800 paper was written in French. | |||
| == Last years and retirement == | |||
| Volta is buried in the city of ]; the '''Tempio Voltiano''' near ] there is a museum devoted to explaining his work; his original instruments and papers are on display there. The building appeared, along with his portrait, on Italian 10.000 ] banknote, before the introduction of the ]. | |||
| ] in 1801.]] | |||
| In 1809, Volta became an associated member of the ].<ref name="dwc" /> In honour of his work, Volta was made a count by ] in 1810.<ref name="pancaldi" /> | |||
| In 1881 an important electrical ], the ], was named in his honor. The ] is named after Volta. Volta Crater on the Moon is also named after him. | |||
| Volta retired in 1819 to his estate in Camnago, a {{lang|it|]}} of ], now named "Camnago Volta" in his honour. He died there on 5 March 1827, just after his 82nd birthday.<ref name="huji" /> Volta's remains were buried in Camnago Volta.<ref name="grave" /> | |||
| == Retirement == | |||
| Volta entered retirement in Spain. | |||
| == |
=== Legacy === | ||
| Volta's legacy is celebrated by the ] memorial located in the public gardens by the lake. There is also a museum that was built in his honour, which exhibits some of the equipment that Volta used to conduct experiments.<ref name="atlas" /> Nearby stands the ], which houses the Voltian Foundation, an organization promoting scientific activities. Volta carried out his experimental studies and produced his first inventions near Como.<ref name="villa" /> ], Aula Volta, 1787, ]]] | |||
| {{reflist}} | |||
| In the ] of the ], there is the classroom (Aula Volta) commissioned by Emperor ] to ] in 1787 for the lectures of Alessandro Volta,<ref name="Aula Volta"/> while in the ] there are many scientific instruments that belonged to Volta and his chair and his blackboard.<ref name="Sala Volta"/> | |||
| His image was depicted on the ] (1990–1997) along with a sketch of his voltaic pile.<ref name="desmond" /> | |||
| ==Gallery== | |||
| <gallery> | |||
| Image:alessandro_volta2.jpg|Alessandro Giuseppe Antonio Anastasio Volta portrait. | |||
| Image:DSC03492.jpg|Volta demonstrating his battery to Napoleon in 1801, by Giuseppe Bertini | |||
| Image:Voltaic pile.jpg|Volta pile on exhibit in the Volta Temple, Como, Italy | |||
| Image:VoltaBattery.JPG|Another Volta pile on exhibit in the Volta Temple, Como, Italy | |||
| </gallery> | |||
| In late 2017, Nvidia announced a new workstation-focused ] ] called ]. | |||
| ==External links== | |||
| * | |||
| * article on Alessando Volta. | |||
| * | |||
| The ] species '']'', described in 2019 and the strongest bioelectricity producer in nature, was named after Volta.<ref name=":0">{{Cite journal|last1=de Santana|first1=C. David|last2=Crampton|first2=William G. R.|last3=Dillman|first3=Casey B.|last4=Frederico|first4=Renata G.|last5=Sabaj|first5=Mark H.|last6=Covain|first6=Raphaël|last7=Ready|first7=Jonathan|last8=Zuanon|first8=Jansen|last9=de Oliveira|first9=Renildo R.|last10=Mendes-Júnior|first10=Raimundo N.|last11=Bastos|first11=Douglas A.|display-authors=2|date=10 September 2019|title=Unexpected species diversity in electric eels with a description of the strongest living bioelectricity generator|url=|journal=Nature Communications|language=en|volume=10|issue=1|pages=4000|bibcode=2019NatCo..10.4000D|doi=10.1038/s41467-019-11690-z|issn=2041-1723|pmc=6736962|pmid=31506444}}</ref> | |||
| {{Commons|Alessandro Giuseppe Antonio Anastasio Volta}} | |||
| == Religious beliefs == | |||
| {{s-start}} | |||
| Volta was raised as a Catholic and for all of his life continued to maintain his belief.<ref name="zenit" /> Because he was not ordained a clergyman as his family expected, he was sometimes accused of being irreligious and some people have speculated about his possible unbelief, stressing that "he did not join the Church",<ref name="davis" /> or that he virtually "ignored the church's call".<ref name="schiffer" /> Nevertheless, he cast out doubts in a declaration of faith in which he said: | |||
| {{s-awards}} | |||
| ] of the University of Pavia]] | |||
| {{s-bef|before=]}} | |||
| <blockquote> | |||
| {{s-ttl|title=]|years=1794}} | |||
| I do not understand how anyone can doubt the sincerity and constancy of my attachment to the religion which I profess, the Roman, Catholic and Apostolic religion in which I was born and brought up, and of which I have always made confession, externally and internally. I have, indeed, and only too often, failed in the performance of those good works which are the mark of a Catholic Christian, and I have been guilty of many sins: but through the special mercy of God I have never, as far as I know, wavered in my faith... In this faith I recognise a pure gift of God, a supernatural grace; but I have not neglected those human means which confirm belief, and overthrow the doubts which at times arise. I studied attentively the grounds and basis of religion, the works of apologists and assailants, the reasons for and against, and I can say that the result of such study is to clothe religion with such a degree of probability, even for the merely natural reason, that every spirit unperverted by sin and passion, every naturally noble spirit must love and accept it. May this confession which has been asked from me and which I willingly give, written and subscribed by my own hand, with authority to show it to whomsoever you will, for I am not ashamed of the Gospel, may it produce some good fruit!<ref name="kneller" /><ref name="epist" /> | |||
| {{s-aft|after=]}} | |||
| </blockquote> | |||
| {{s-end}} | |||
| == Publications == | |||
| <!-- Metadata: see ] --> | |||
| ] | |||
| *{{cite book |last=Voltae |first=Alexandri |display-authors=0 |title=De vi attractiva ignis electrici, ac phaenomenis inde pendentibus |trans-title=The attractive force of an electric fire and the resulting phenomena |location=Novo Comi |publisher=Typis Octavii Staurenghi |year=1769 |oclc=1419897 |language=la |ref=none |url=https://play.google.com/books/reader?id=Ha9gAAAAcAAJ&pg=GBS.PP10}} | |||
| ===Lesser known collections=== | |||
| {{Persondata | |||
| * ''Briefe über thierische elektricität'' (1900) (''Letters about thieric electricity'', Available through Worldcat.org libraries, Leipzig, W. Engelmann, publisher) | |||
| |NAME= Volta, Alessandro | |||
| * ''Untersuchungen über den Galvanismus'', 1796 bis 1800 (''Studies on Galvanism'', Available through Worldcat.org libraries) | |||
| |ALTERNATIVE NAMES= | |||
| * ''Del modo di render sensibilissima la più debole elettricità sia naturale, sia artificiale'' (''Of the method of rendering very sensible the weakest natural or artificial electricity'' By Alexander Volta, Professor Of Experimental Philosophy In Como, &c. Read at the Royal Society, 14 March 1782, Held in WorldCat libraries) | |||
| |SHORT DESCRIPTION= ] | |||
| |DATE OF BIRTH= {{birth date|1745|2|18|mf=y}} | |||
| == See also == | |||
| |PLACE OF BIRTH= ], ], ] | |||
| * ] | |||
| |DATE OF DEATH= {{death date|1827|3|5|mf=y}} | |||
| * ] | |||
| |PLACE OF DEATH= ], ], ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| == References == | |||
| {{reflist|colwidth=30em|refs= | |||
| <ref name="atlas">{{cite book |title=The Geek Atlas: 128 Places Where Science and Technology Come Alive |first=John |last=Graham-Cumming |chapter=Tempio Voltiano, Como, Italy |publisher=O'Reilly Media |year=2009 |isbn=9780596523206 |page=95 |chapter-url=https://books.google.com/books?id=HhEC0q-O1ewC&pg=PA95}}</ref> | |||
| <ref name="berzolari">Alberto Gigli Berzolari, ''"Volta's Teaching in Como and Pavia"'' - Nuova voltiana</ref> | |||
| <ref name="davis">'Adam-Hart Davis. (2012). ''Engineers''. Penguin. p. 138</ref> | |||
| <ref name="desmond">{{cite book|last=Desmond|first=Kevin|title=Innovators in Battery Technology: Profiles of 95 Influential Electrochemists|url=https://books.google.com/books?id=gliiDAAAQBAJ&pg=PA235|year=2016|publisher=McFarland|isbn=9781476622781|page=235}}</ref> | |||
| <ref name="dwc">{{cite web |url=http://www.dwc.knaw.nl/biografie/pmknaw/?pagetype=authorDetail&aId=PE00003610 |title=Alessandro G.A.A. Volta (1745–1827) |publisher=Royal Netherlands Academy of Arts and Sciences |access-date=20 July 2015 |archive-url=https://web.archive.org/web/20150903182941/http://www.dwc.knaw.nl/biografie/pmknaw/?pagetype=authorDetail&aId=PE00003610 |archive-date=3 September 2015 |url-status=dead }}</ref> | |||
| <ref name="edison">, Edison.</ref> | |||
| <ref name="elliott">{{cite journal|author=Elliott, P. |title=Abraham Bennet F.R.S. (1749–1799): a provincial electrician in eighteenth-century England |journal=Notes and Records of the Royal Society of London |volume=53 |issue=1 |pages=59–78 |year=1999 |url=http://www.journals.royalsoc.ac.uk/content/klgdd0umcmvjqnpr/fulltext.pdf |archive-url=https://ghostarchive.org/archive/20221009/http://www.journals.royalsoc.ac.uk/content/klgdd0umcmvjqnpr/fulltext.pdf |archive-date=9 October 2022 |url-status=live |doi=10.1098/rsnr.1999.0063|s2cid=144062032 }}{{dead link|date=June 2017 |bot=InternetArchiveBot |fix-attempted=yes}}</ref> | |||
| <ref name="epist">Alessandro Volta. 1955. ''''. Zanichelli. p. 29</ref> | |||
| <ref name="grave">For a photograph of his gravesite, and other Volta locales, see {{cite web |title=Volta's localities |url=http://www.corrieredicomo.it/pg_interna.cfm?IndiceID=526&MenuID=2 |access-date=20 June 2009}} {{Dead link|date=September 2010|bot=H3llBot}}</ref> | |||
| <ref name="huji">{{cite web |url=http://chem.ch.huji.ac.il/history/volta.htm#end |title=Volta |publisher=Institute of Chemistry – Jerusalem |access-date=1 May 2009 |archive-url= https://web.archive.org/web/20090408220853/http://chem.ch.huji.ac.il/history/volta.htm |archive-date= 8 April 2009 | url-status= live}}</ref> | |||
| <ref name="ieeeghn">{{cite web |title=Milestones:Volta's Electrical Battery Invention, 1799 |url=http://ethw.org/Milestones:Volta's_Electrical_Battery_Invention,_1799 |website=ETHW|date=12 February 2020 |access-date=4 December 2021}}</ref> | |||
| <ref name="kneller">Kneller, Karl Alois, '''' (1911), p. 117–118</ref> | |||
| <ref name="lifeworks">{{cite web |url=http://www.alessandrovolta.info/life_and_works_8.html |title=Life and works |newspaper=Alessandrovolta.info |publisher=Editoriale srl |location=Como, Italy |access-date=18 February 2015 |archive-url=https://web.archive.org/web/20150221032105/http://www.alessandrovolta.info/life_and_works_8.html |archive-date=21 February 2015 |url-status=dead}}</ref> | |||
| <ref name="marsh">Alessandro Volta, ''Lettere del Signor Don Alessandro Volta ... Sull' Aria Inflammabile Nativa delle Paludi'' (Milan, (Italy): Giuseppe Marelli, 1777).</ref> | |||
| <ref name="methane">{{cite book |url=http://www.bookrags.com/research/methane-woc/ |title=Methane |publisher=BookRags |access-date=26 January 2012}}</ref> | |||
| <ref name="munro">{{cite book|author=Munro, John|title=Pioneers of Electricity; Or, Short Lives of the Great Electricians|year=1902|publisher=The Religious Tract Society| location = London|pages=–102|url=https://archive.org/details/pioneerselectri00munrgoog}}</ref> | |||
| <ref name="pancaldi">{{cite book|last=Pancaldi|first=Giuliano|year= 2003|title=Volta, Science and Culture in the Age of Enlightenment|publisher=Princeton Univ. Press|url=https://books.google.com/books?id=hGoYB1Twx4sC|isbn=978-0-691-12226-7}}</ref> | |||
| <ref name="routledge">{{cite book|title=A popular history of science|author=Robert Routledge|url=https://archive.org/details/b24869880|page= |edition=2nd|year=1881|publisher=G. Routledge and Sons}}</ref> | |||
| <ref name="royal">{{cite web|title=Enterprise and electrolysis|url=http://www.rsc.org/chemistryworld/Issues/2003/August/electrolysis.asp|website=rsc.org|publisher=Royal Society of Chemistry|access-date=18 February 2015}}</ref> | |||
| <ref name="schiffer">Michael Brian Schiffer (2003), ''Draw the Lightning Down: Benjamin Franklin and Electrical Technology in the Age of Enlightenment''. University of California Press. p. 55</ref> | |||
| <ref name="villa">{{cite web | url=http://www.centrovolta.it/volta-en/index.php?option=com_content&task=view&id=63 | title=Villa Olmo | website=centrovolta.it | archive-url=https://web.archive.org/web/20090210083231/http://www.centrovolta.it/volta-en/index.php?option=com_content&task=view&id=63 | archive-date=10 February 2009}}</ref> | |||
| <ref name="wilcke">Joh. Carl Wilcke (1762) "Ytterligare rön och försök om ''contraira electriciteterne'' vid laddningen och därtil hörande delar" (Additional findings and experiments on the opposing electric charges during charging, and parts related thereto) ''Kongliga Svenska Vetenskaps Academiens Handlingar'' (Proceedings of the Royal Swedish Science Academy), vol. 23, pages , 245–266.</ref> | |||
| <ref name="williams">{{cite book |author=Williams, Jeffrey Huw |title=Defining and Measuring Nature: The Make of All Things |year=2014 |publisher=Morgan & Claypool |url=https://books.google.com/books?id=iPO9BAAAQBAJ&q=volta%27s+law+of+capacitance&pg=PT76 |isbn=978-1-627-05278-8}}</ref> | |||
| <ref name="zenit">{{cite web|url=http://www.zenit.org/article-29971?l=italian|archive-url=https://archive.today/20130416082708/http://www.zenit.org/article-29971?l=italian|url-status=dead|archive-date=16 April 2013|title=Gli scienziati cattolici che hanno fatto lItalia (Catholic scientists who made Italy)|publisher=Zenit}}</ref> | |||
| }} | }} | ||
| {{DEFAULTSORT:Volta}} | |||
| == External links == | |||
| {{Commons category|Alessandro Volta}} | |||
| * {{CathEncy|wstitle=Alessandro Volta}} | |||
| * | |||
| * {{Webarchive|url=https://web.archive.org/web/20100102131114/http://ideafinder.com/history/inventors/volta.htm |date=2 January 2010 }} | |||
| * | |||
| * {{Cite EB1911|wstitle=Volta, Alessandro |volume=28 |page=198}} | |||
| * | |||
| * | |||
| * | |||
| {{Copley Medallists 1751–1800}} | |||
| {{Scientists whose names are used as SI units}} | |||
| {{Authority control}} | |||
| {{DEFAULTSORT:Volta, Alessandro}} | |||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 19:49, 9 December 2024
Italian physicist and chemist (1745–1827) For the concept car, see Toyota Alessandro Volta.
| CountAlessandro VoltaForMemRS | |
|---|---|
 | |
| Born | Alessandro Giuseppe Antonio Anastasio Volta (1745-02-18)18 February 1745 Como, Duchy of Milan |
| Died | 5 March 1827(1827-03-05) (aged 82) Como, Lombardy-Venetia, Austrian Empire |
| Known for | Inventing the electric battery Discovering methane |
| Spouse |
Teresa Peregrini (m. 1794) |
| Children | 3 |
| Awards |
|
| Scientific career | |
| Fields | Physics Chemistry |
| Institutions | University of Pavia |
Alessandro Giuseppe Antonio Anastasio Volta (UK: /ˈvɒltə/, US: /ˈvoʊltə/; Italian: [alesˈsandro ˈvɔlta]; 18 February 1745 – 5 March 1827) was an Italian physicist and chemist who was a pioneer of electricity and power, and is credited as the inventor of the electric battery and the discoverer of methane. He invented the voltaic pile in 1799, and reported the results of his experiments in a two-part letter to the president of the Royal Society, which was published in 1800. With this invention, Volta proved that electricity could be generated chemically and debunked the prevalent theory that electricity was generated solely by living beings. Volta's invention sparked a great amount of scientific excitement and led others to conduct similar experiments, which eventually led to the development of the field of electrochemistry.
Volta drew admiration from Napoleon Bonaparte for his invention, and was invited to the Institute of France to demonstrate his invention to the members of the institute. Throughout his life, Volta enjoyed a certain amount of closeness with the emperor who conferred upon him numerous honours. Volta held the chair of experimental physics at the University of Pavia for nearly 40 years and was widely idolised by his students. Despite his professional success, Volta was inclined towards domestic life and this was more apparent in his later years when he tended to live secluded from public life and more for the sake of his family. He died in 1827 from a series of illnesses which began in 1823. The SI unit of electric potential is named the volt in his honour.
Early life and marriage
Volta was born in Como, a town in northern Italy, on 18 February 1745. His father, Filippo Volta, was of noble lineage. His mother, Donna Maddalena, came from the family of the Inzaghis. In 1794, Volta married an aristocratic lady also from Como, Teresa Peregrini, with whom he raised three sons: Zanino, Flaminio, and Luigi.
Career
In 1774, he became a professor of physics at the Royal School in Como. A year later, he improved and popularised the electrophorus, a device that produced static electricity. His promotion of it was so extensive that he is often credited with its invention, even though a machine operating on the same principle was described in 1762 by the Swedish experimenter Johan Wilcke. In 1777, he travelled through Switzerland, where he befriended H. B. de Saussure.
In the years between 1776 and 1778, Volta studied the chemistry of gases. He researched and discovered methane after reading a paper by Benjamin Franklin of the United States on "flammable air". In November 1776, he found methane in the marshes of Angera on Lake Maggiore, and by 1778 he managed to isolate it. He devised experiments such as the ignition of methane by an electric spark in a closed vessel.
Volta also studied what we now call electrical capacitance, developing separate means to study both electrical potential difference (V) and charge (Q), and discovering that for a given object, they are proportional. This is called Volta's Law of Capacitance, and for this work, the unit of electrical potential has been named the volt.
In 1779, he became a professor of experimental physics at the University of Pavia, a chair that he occupied for almost 40 years. Volta's lectures were so crowded with students that the subsequent emperor Joseph II ordered the construction (based on a project by Leopold Pollack) of a new "physical theatre", today the "Aula Volta". Furthermore, the emperor granted Volta substantial funding to equip the physics cabinet with instruments, purchased by Volta in England and France. At the University History Museum of the University of Pavia there are 150 of them, used by Alessandro Volta.
Volta and Galvani

Luigi Galvani, an Italian physicist, discovered something he named "animal electricity" when two different metals were connected in series with a frog's leg and to one another. Volta realised that the frog's leg served as both a conductor of electricity (what we would now call an electrolyte) and as a detector of electricity. He also understood that the frog's legs were irrelevant to the electric current, which was caused by the two differing metals. He replaced the frog's leg with brine-soaked paper and detected the flow of electricity by other means familiar to him from his previous studies. In this way, he discovered the electrochemical series, and the law that the electromotive force (emf) of a galvanic cell, consisting of a pair of metal electrodes separated by electrolyte, is the difference between their two electrode potentials (thus, two identical electrodes and a common electrolyte give zero net emf). This may be called Volta's Law of the electrochemical series.
In 1800, as the result of a professional disagreement over the galvanic response advocated by Galvani, Volta invented the voltaic pile, an early electric battery, which produced a steady electric current. Volta had determined that the most effective pair of dissimilar metals to produce electricity was zinc and copper. Initially, he experimented with individual cells in series, each cell being a wine goblet filled with brine into which the two dissimilar electrodes were dipped. The voltaic pile replaced the goblets with cardboard soaked in brine.
Early battery
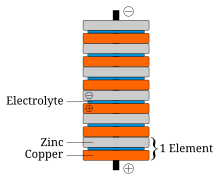
In announcing his discovery of the voltaic pile, Volta paid tribute to the influences of William Nicholson, Tiberius Cavallo, and Abraham Bennet.
The battery made by Volta is credited as one of the first electrochemical cells. It consists of two electrodes: one made of zinc, the other of copper. The electrolyte is either sulfuric acid mixed with water or a form of saltwater brine. The electrolyte exists in the form 2 H and SO2−4. Zinc metal, which is higher in the electrochemical series than both copper and hydrogen, is oxidized to zinc cations (Zn) and creates electrons that move to the copper electrode. The positively charged hydrogen ions (protons) capture electrons from the copper electrode, forming bubbles of hydrogen gas, H2. This makes the zinc rod the negative electrode and the copper rod the positive electrode. Thus, there are two terminals, and an electric current will flow if they are connected. The chemical reactions in this voltaic cell are as follows:
- Zinc:
- Zn → Zn + 2e
- Sulfuric acid:
- 2H + 2e → H2
Copper metal does not react, but rather it functions as a catalyst for the hydrogen-gas formation and an electrode for the electric current. The sulfate anion (SO2−4) does not undergo any chemical reaction either, but migrates to the zinc anode to compensate for the charge of the zinc cations formed there. However, this cell also has some disadvantages. It is unsafe to handle, since sulfuric acid, even if diluted, can be hazardous. Also, the power of the cell diminishes over time because the hydrogen gas is not released. Instead, it accumulates on the surface of the copper electrode and forms a barrier between the metal and the electrolyte solution.
Last years and retirement

In 1809, Volta became an associated member of the Royal Institute of the Netherlands. In honour of his work, Volta was made a count by Napoleon Bonaparte in 1810.
Volta retired in 1819 to his estate in Camnago, a frazione of Como, Italy, now named "Camnago Volta" in his honour. He died there on 5 March 1827, just after his 82nd birthday. Volta's remains were buried in Camnago Volta.
Legacy
Volta's legacy is celebrated by the Tempio Voltiano memorial located in the public gardens by the lake. There is also a museum that was built in his honour, which exhibits some of the equipment that Volta used to conduct experiments. Nearby stands the Villa Olmo, which houses the Voltian Foundation, an organization promoting scientific activities. Volta carried out his experimental studies and produced his first inventions near Como.

In the Old Campus of the University of Pavia, there is the classroom (Aula Volta) commissioned by Emperor Joseph II to Leopoldo Pollack in 1787 for the lectures of Alessandro Volta, while in the University History Museum there are many scientific instruments that belonged to Volta and his chair and his blackboard.
His image was depicted on the Italian Lire 10,000 note (1990–1997) along with a sketch of his voltaic pile.
In late 2017, Nvidia announced a new workstation-focused GPU microarchitecture called Volta.
The electric eel species Electrophorus voltai, described in 2019 and the strongest bioelectricity producer in nature, was named after Volta.
Religious beliefs
Volta was raised as a Catholic and for all of his life continued to maintain his belief. Because he was not ordained a clergyman as his family expected, he was sometimes accused of being irreligious and some people have speculated about his possible unbelief, stressing that "he did not join the Church", or that he virtually "ignored the church's call". Nevertheless, he cast out doubts in a declaration of faith in which he said:

I do not understand how anyone can doubt the sincerity and constancy of my attachment to the religion which I profess, the Roman, Catholic and Apostolic religion in which I was born and brought up, and of which I have always made confession, externally and internally. I have, indeed, and only too often, failed in the performance of those good works which are the mark of a Catholic Christian, and I have been guilty of many sins: but through the special mercy of God I have never, as far as I know, wavered in my faith... In this faith I recognise a pure gift of God, a supernatural grace; but I have not neglected those human means which confirm belief, and overthrow the doubts which at times arise. I studied attentively the grounds and basis of religion, the works of apologists and assailants, the reasons for and against, and I can say that the result of such study is to clothe religion with such a degree of probability, even for the merely natural reason, that every spirit unperverted by sin and passion, every naturally noble spirit must love and accept it. May this confession which has been asked from me and which I willingly give, written and subscribed by my own hand, with authority to show it to whomsoever you will, for I am not ashamed of the Gospel, may it produce some good fruit!
Publications

- De vi attractiva ignis electrici, ac phaenomenis inde pendentibus [The attractive force of an electric fire and the resulting phenomena] (in Latin). Novo Comi: Typis Octavii Staurenghi. 1769. OCLC 1419897.
Lesser known collections
- Briefe über thierische elektricität (1900) (Letters about thieric electricity, Available through Worldcat.org libraries, Leipzig, W. Engelmann, publisher)
- Untersuchungen über den Galvanismus, 1796 bis 1800 (Studies on Galvanism, Available through Worldcat.org libraries)
- Del modo di render sensibilissima la più debole elettricità sia naturale, sia artificiale (Of the method of rendering very sensible the weakest natural or artificial electricity By Alexander Volta, Professor Of Experimental Philosophy In Como, &c. Read at the Royal Society, 14 March 1782, Held in WorldCat libraries)
See also
- Armstrong effect
- Electrophorus
- History of the battery
- History of the internal combustion engine
- Lemon battery
- Mercury beating heart
- Thermoelectric effect
- Volta (lunar crater)
- Volta Prize
References
- ^ Pancaldi, Giuliano (2003). Volta, Science and Culture in the Age of Enlightenment. Princeton Univ. Press. ISBN 978-0-691-12226-7.
- Alberto Gigli Berzolari, "Volta's Teaching in Como and Pavia" - Nuova voltiana
- Hall of Fame, Edison.
- "Milestones:Volta's Electrical Battery Invention, 1799". ETHW. 12 February 2020. Retrieved 4 December 2021.
- ^ "Enterprise and electrolysis". rsc.org. Royal Society of Chemistry. Retrieved 18 February 2015.
- Volta, Alessandro (20 March 1800). Banks, Joseph (ed.). "On the Electricity excited by the mere Contact of conducting Substances of different kinds". Philosophical Transactions of the Royal Society (in French). 90: 403–431. doi:10.1098/rstl.1800.0018.
- ^ Munro, John (1902). Pioneers of Electricity; Or, Short Lives of the Great Electricians. London: The Religious Tract Society. pp. 89–102.
- "Life and works". Alessandrovolta.info. Como, Italy: Editoriale srl. Archived from the original on 21 February 2015. Retrieved 18 February 2015.
- Joh. Carl Wilcke (1762) "Ytterligare rön och försök om contraira electriciteterne vid laddningen och därtil hörande delar" (Additional findings and experiments on the opposing electric charges during charging, and parts related thereto) Kongliga Svenska Vetenskaps Academiens Handlingar (Proceedings of the Royal Swedish Science Academy), vol. 23, pages 206–229, 245–266.
- Alessandro Volta, Lettere del Signor Don Alessandro Volta ... Sull' Aria Inflammabile Nativa delle Paludi (Milan, (Italy): Giuseppe Marelli, 1777).
- Methane. BookRags. Retrieved 26 January 2012.
- ^ Williams, Jeffrey Huw (2014). Defining and Measuring Nature: The Make of All Things. Morgan & Claypool. ISBN 978-1-627-05278-8.
- ^ "Aula Volta". Luoghi Voltiani. Retrieved 21 August 2022.
- ^ "Sala Volta". Musei Unipv. Retrieved 21 August 2022.
- "Gabinetto Fisico". Luoghi Voltiani. Retrieved 21 August 2022.
- Price, Derek deSolla (1982). On the Brink of Tomorrow: Frontiers of Science. Washington D.C.: National Geographic Society. pp. 16–17.
- Robert Routledge (1881). A popular history of science (2nd ed.). G. Routledge and Sons. p. 553.
- Elliott, P. (1999). "Abraham Bennet F.R.S. (1749–1799): a provincial electrician in eighteenth-century England" (PDF). Notes and Records of the Royal Society of London. 53 (1): 59–78. doi:10.1098/rsnr.1999.0063. S2CID 144062032. Archived (PDF) from the original on 9 October 2022.
- "Alessandro G.A.A. Volta (1745–1827)". Royal Netherlands Academy of Arts and Sciences. Archived from the original on 3 September 2015. Retrieved 20 July 2015.
- "Volta". Institute of Chemistry – Jerusalem. Archived from the original on 8 April 2009. Retrieved 1 May 2009.
- For a photograph of his gravesite, and other Volta locales, see "Volta's localities". Retrieved 20 June 2009.
- Graham-Cumming, John (2009). "Tempio Voltiano, Como, Italy". The Geek Atlas: 128 Places Where Science and Technology Come Alive. O'Reilly Media. p. 95. ISBN 9780596523206.
- "Villa Olmo". centrovolta.it. Archived from the original on 10 February 2009.
- Desmond, Kevin (2016). Innovators in Battery Technology: Profiles of 95 Influential Electrochemists. McFarland. p. 235. ISBN 9781476622781.
- de Santana, C. David; Crampton, William G. R.; et al. (10 September 2019). "Unexpected species diversity in electric eels with a description of the strongest living bioelectricity generator". Nature Communications. 10 (1): 4000. Bibcode:2019NatCo..10.4000D. doi:10.1038/s41467-019-11690-z. ISSN 2041-1723. PMC 6736962. PMID 31506444.
- "Gli scienziati cattolici che hanno fatto lItalia (Catholic scientists who made Italy)". Zenit. Archived from the original on 16 April 2013.
- 'Adam-Hart Davis. (2012). Engineers. Penguin. p. 138
- Michael Brian Schiffer (2003), Draw the Lightning Down: Benjamin Franklin and Electrical Technology in the Age of Enlightenment. University of California Press. p. 55
- Kneller, Karl Alois, Christianity and the leaders of modern science; a contribution to the history of culture in the nineteenth century (1911), p. 117–118
- Alessandro Volta. 1955. Epistolario, Volume 5. Zanichelli. p. 29
External links
- Herbermann, Charles, ed. (1913). "Alessandro Volta" . Catholic Encyclopedia. New York: Robert Appleton Company.
- Volta and the "Pile"
- Alessandro Volta Archived 2 January 2010 at the Wayback Machine
- Count Alessandro Volta
- Chisholm, Hugh, ed. (1911). "Volta, Alessandro" . Encyclopædia Britannica. Vol. 28 (11th ed.). Cambridge University Press. p. 198.
- Electrical units history.
- Life of Alessandro Volta: Biography; Inventions; Facts
- Alessandro Volta | Biography, Facts, Battery, & Invention | Britannica
| Copley Medallists (1751–1800) | |
|---|---|
|
- Alessandro Volta
- 1745 births
- 1827 deaths
- People from Como
- 19th-century Italian physicists
- Battery inventors
- Enlightenment scientists
- Fellows of the Royal Society
- Independent scientists
- History of neuroscience
- 18th-century Italian inventors
- 18th-century Italian physicists
- Italian Roman Catholics
- Members of the Royal Netherlands Academy of Arts and Sciences
- People associated with electricity
- Recipients of the Copley Medal
- Italian scientific instrument makers
- Academic staff of the University of Pavia
