| Revision as of 11:41, 26 May 2010 editLfh (talk | contribs)Autopatrolled, Extended confirmed users6,280 edits a class, not a compound← Previous edit | Latest revision as of 04:27, 24 December 2024 edit undoThe Blade of the Northern Lights (talk | contribs)Edit filter managers, Autopatrolled, Oversighters, Administrators55,776 edits →Toxicity: Who would have thought? | ||
| (679 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{short description|Any molecule with a cyano group (–C≡N)}} | |||
| {{About|the class of chemical compounds}} | {{About|the class of chemical compounds}} | ||
| {{distinguish|Nitrile}} | |||
| {{semiprotected|small=yes}} | |||
| {{Chembox | |||
| | ImageFile = Cyanide-montage.png | |||
| | ImageAlt = Space-filling model of the cyanide anion: carbon bound to smaller nitrogen atom | |||
| | Name= | |||
| | PIN = | |||
| | SystematicName = Nitridocarbonate(II) | |||
| | IUPACName = | |||
| | OtherNames = | |||
| |Section1 = {{Chembox Identifiers | |||
| | CASNo = 57-12-5 | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = OXN4E7L11K | |||
| | PubChem = 5975 | |||
| | ChEBI = 17514 | |||
| | SMILES = #N | |||
| | ChemSpiderID = 5755 | |||
| | InChI = 1S/CN/c1-2/q-1 | |||
| | InChIKey = XFXPMWWXUTWYJX-UHFFFAOYSA-N | |||
| }} | |||
| |Section2 = {{Chembox Properties | |||
| | Formula = {{chem2|CN−}} | |||
| | C=1|N=1 | |||
| | Appearance = | |||
| | Solubility = | |||
| | ConjugateAcid = ]}} | |||
| |Section3 = {{Chembox Hazards | |||
| | MainHazards = The cyanide ion {{chem2|CN−}} is one of the most poisonous chemicals. It may cause death in minutes. | |||
| | FlashPt = | |||
| | AutoignitionPt = }} | |||
| }} | |||
| In ], '''cyanide''' ({{ety|el|kyanos|]}}) is a ] that contains a {{chem2|C\tN}} ]. This group, known as the '''cyano group''', consists of a ] atom ]ed to a ] atom.<ref>{{cite journal|url=http://goldbook.iupac.org/C01486.html |title=cyanides|website=] |date=2014 |doi=10.1351/goldbook.C01486 |doi-access=free }}</ref> | |||
| [[Image:Cyanide-montage.png|thumb|right|150px|The '''cyanide''' ion, CN<sup>−</sup>.<br> | |||
| From the top:<br> | |||
| 1. Valence-bond structure<br> | |||
| 2. ]<br> | |||
| 3. Electrostatic potential surface<br> | |||
| 4. 'Carbon lone pair' ]]] | |||
| A '''cyanide''' is any ] that contains the ] (C≡N), which consists of a ] ] ]ed to a ] atom. Inorganic cyanides are generally salts of the ] CN<sup>−</sup>.<ref>Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.</ref><ref>G. L. Miessler and D. A. Tarr “Inorganic Chemistry” 3rd Ed, Pearson/Prentice Hall publisher, ISBN 0-13-035471-6.</ref> ]s that have a –C≡N ] are called ]s. Of the many kinds of cyanide compounds, some are gases; others are solids or liquids. Those that can release the cyanide ] CN<sup>−</sup> are highly toxic to animals.<ref name="CMC">{{Cite web|url=http://www.cyanidecode.org/cyanide_environmental.php| title=Environmental and Health Effects of Cyanide| publisher=]|year=2006| accessdate=4 August 2009}}</ref> | |||
| In ] cyanides, the cyanide group is present as the cyanide anion {{chem2|−C\tN}}. This anion is ]. Soluble ] such as ] (NaCN) and ] (KCN) are highly toxic.<ref name="CMC">{{Cite web| url=http://www.cyanidecode.org/cyanide_environmental.php| title=Environmental and Health Effects of Cyanide| publisher=International Cyanide Management Institute| year=2006| access-date=4 August 2009| archive-date=30 November 2012| archive-url=https://web.archive.org/web/20121130094124/http://www.cyanidecode.org/cyanide_environmental.php| url-status=dead}}</ref> ], also known as hydrogen cyanide, or HCN, is a highly ] liquid that is produced on a large scale industrially. It is obtained by acidification of cyanide salts. | |||
| An example of a nitrile is CH<sub>3</sub>CN, ] (ethanenitrile per ]), also known as ] cyanide. Nitriles do not release cyanide ions. A functional group with a hydroxyl and cyanide bonded to the same carbon is called ], and cyanohydridins are hydrolyzed into ] and a carbonyl compound (ketone or aldehyde). | |||
| ] cyanides are usually called ]s. In nitriles, the {{chem2|\sC\tN}} group is linked by a single ] to carbon. For example, in ] ({{chem2|CH3\sC\tN}}), the cyanide group is bonded to ] ({{chem2|\sCH3}}). Although nitriles generally do not release cyanide ions, the ]s do and are thus toxic. | |||
| ==Etymology== | |||
| The word "cyanide" was derived from "]", a cyanide derivative of ]. Iron cyanides were first discovered as components on the intensely colored dye ]. ''Kyaneos'' is Greek for "(dark) blue".<ref>{{cite book |last=Senning |first=Alexander |title=Elsevier's Dictionary of Chemoetymology |year=2006 |publisher=Elsevier |isbn=0444522395}}</ref> | |||
| ==Bonding== | |||
| The cyanide ion {{chem2|−C\tN}} is ] with ] {{chem2|-C\tO+}} and with molecular ] N≡N. A triple bond exists between C and N. The negative charge is concentrated on ] C.<ref>Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. {{ISBN|0-7506-3365-4}}.{{page needed|date=July 2015}}</ref><ref>G. L. Miessler and D. A. Tarr "Inorganic Chemistry" 3rd Ed, Pearson/Prentice Hall publisher, {{ISBN|0-13-035471-6}}.{{page needed|date=July 2015}}</ref> | |||
| ==Occurrence== | ==Occurrence== | ||
| Cyanides are produced by certain ], ], and ] and are found in a number of foods and plants. Cyanides are found, although in small amounts, in certain seeds and stones, e.g. those of ], ], ], and ].<ref>{{cite web |url=http://www.atsdr.cdc.gov/tfacts8.html |title=ToxFAQs for Cyanide |accessdate=2008-06-28 |date=July 2006 |publisher= | |||
| ]}}</ref> In plants, cyanides are usually bound to ] molecules in the form of cyanogenic ]s and defend the plant against ]s. ] roots (also called manioc), an important ]-like food grown in tropical countries (and the base from which ] is made), also contain cyanogenic glycosides.<ref>{{cite journal |first=J. |last=Vetter |title=Plant cyanogenic glycosides |journal=Toxicon |year=2000 |volume=38 |pages=11–36 |doi=10.1016/S0041-0101(99)00128-2 |pmid=10669009 |issue=1}}</ref><ref name=jones>{{cite journal |first=D. A. |last=Jones |title= Why are so many food plants cyanogenic? |journal=] |year=1998 |volume=47 |pages=155–162 |doi=10.1016/S0031-9422(97)00425-1 |pmid=9431670 |issue=2}}</ref> | |||
| ===In nature=== | |||
| The ] CN· has been identified in interstellar space.<ref>{{cite paper |last=Pieniazek |first=Piotr A. |coauthors=Bradforth, Stephen E.; Krylov, Anna I. |title=Spectroscopy of the Cyano Radical in an Aqueous Environment |date=2005-12-07 |publisher=Department of Chemistry, ] |location=], ] 90089-0482 |url=http://www-bcf.usc.edu/~krylov/pubs/pdf/jpca-110-4854.pdf |format=PDF |doi=10.1021/jp0545952}}</ref> | |||
| ] in ].]] | |||
| Cyanides are produced by certain ], ], and ]. It is an ] in a number of plants. Cyanides are found in substantial amounts in certain seeds and fruit stones, e.g., those of ]s, ]s, ]s, and ]es.<ref>{{Cite web|url=https://wwwn.cdc.gov/TSP/ToxFAQs/ToxFAQsLanding.aspx?id=71&tid=19 |title=ToxFAQs for Cyanide |access-date=2008-06-28 |date = July 2006|publisher=]}}</ref> Chemical compounds that can release cyanide are known as cyanogenic compounds. In plants, cyanides are usually bound to ] molecules in the form of cyanogenic ]s and defend the plant against ]s. ] roots (also called manioc), an important ]-like food grown in tropical countries (and the base from which ] is made), also contain cyanogenic glycosides.<ref>{{Cite journal|first=J. |last=Vetter |title=Plant cyanogenic glycosides |journal=Toxicon |year=2000 |volume=38 |pages=11–36 |doi=10.1016/S0041-0101(99)00128-2 |pmid=10669009 |issue=1}}</ref><ref name=jones>{{Cite journal|first=D. A. |last=Jones |title= Why are so many food plants cyanogenic? |journal=] |year=1998 |volume=47 |pages=155–162 |doi=10.1016/S0031-9422(97)00425-1 |pmid=9431670 |issue=2|bibcode=1998PChem..47..155J }}</ref> | |||
| The ] bamboo '']'' produces cyanide as a deterrent to grazing. In response, the ], which eats the bamboo, has developed a high tolerance to cyanide. | |||
| Hydrogen cyanide is produced by the combustion or ] of certain materials under oxygen-deficient conditions. For example it can be detected in the ] of ]s and ] smoke. Certain ]s, especially those derived from ], release hydrogen cyanide when heated or burnt.<ref name="CDC">{{cite web|url=http://www.bt.cdc.gov/Agent/cyanide/basics/facts.asp|title=Facts about cyanide:Where cyanide is found and how it is used|last=Anon|date=27 January 2004|work=CDC Emergency preparedness and response|publisher=Centers for Disease Control and Prevention|accessdate=13 April 2010}}</ref> | |||
| The ] enzymes contain cyanide ]s attached to iron in their active sites. The biosynthesis of cyanide in the ]s proceeds from ], which converts to ]yl ], the {{chem2|CN−}} donor.<ref>{{cite journal |last1=Reissmann |first1=Stefanie |last2=Hochleitner |first2=Elisabeth |last3=Wang |first3=Haofan |last4=Paschos |first4=Athanasios |last5=Lottspeich |first5=Friedrich |last6=Glass |first6=Richard S. |last7=Böck |first7=August |title=Taming of a Poison: Biosynthesis of the NiFe-Hydrogenase Cyanide Ligands |journal=Science |volume=299 |issue=5609 |pages=1067–1070 |year=2003 |pmid=12586941 |doi=10.1126/science.1080972 |bibcode=2003Sci...299.1067R |s2cid=20488694 |url=http://pdfs.semanticscholar.org/d359/5a5928df6c6209f88e105c937ccce0a05237.pdf |archive-url=https://web.archive.org/web/20201123134841/http://pdfs.semanticscholar.org/d359/5a5928df6c6209f88e105c937ccce0a05237.pdf |archive-date=2020-11-23 |url-status=live }}</ref> | |||
| ===Coordination chemistry=== | |||
| The cyanide anion is a potent ] for many transition metals.<ref>Sharpe, A. G. The Chemistry of Cyano Complexes of the Transition Metals; Academic Press: London, 1976</ref> The very high affinities of metals for this ] can be attributed to its negative charge, compactness, and ability to engage in π-bonding. Well known complexes include: | |||
| *hexacyanides <sup>3−</sup> (M = Ti, V, Cr, Mn, Fe, Co), which are octahedral in shape; | |||
| *the tetracyanides, <sup>2−</sup> (M = Ni, Pd, Pt), which are square planar in their geometry; | |||
| *the dicyanides <sup>−</sup> (M = Cu, Ag, Au), which are linear in geometry. | |||
| ===Interstellar medium=== | |||
| The deep ] pigment ], used in the making of ]s, is derived from ] cyanide complexes (hence the name ''cyanide'', from ], a shade of blue). Prussian blue can produce hydrogen cyanide when exposed to strong acids. | |||
| The ] <sup>•</sup>CN has been identified in ].<ref>{{Cite journal |last=Pieniazek |first=Piotr A. |author2=Bradforth, Stephen E. |author3=Krylov, Anna I. |title=Spectroscopy of the Cyano Radical in an Aqueous Environment |date=2005-12-07 |pages=4854–4865 |issue=14 |volume=110 |url=http://www-bcf.usc.edu/~krylov/pubs/pdf/jpca-110-4854.pdf |journal=The Journal of Physical Chemistry A |pmid=16599455 |doi=10.1021/jp0545952 |bibcode=2006JPCA..110.4854P |access-date=2008-08-23 |archive-url=https://web.archive.org/web/20080911131555/http://www-bcf.usc.edu/~krylov/pubs/pdf/jpca-110-4854.pdf |archive-date=2008-09-11 |url-status=dead }}</ref> ], {{chem2|(CN)2}}, is used to measure the temperature of ].<ref>{{cite journal |title = Interstellar Cyanogen and the Temperature of the Cosmic Microwave Background Radiation |author1=Roth, K. C. |author2=Meyer, D. M. |author3=Hawkins, I.|author3-link=Isabel Hawkins |journal = The Astrophysical Journal |year = 1993 |volume = 413 |issue = 2 |pages = L67–L71 |doi = 10.1086/186961 |bibcode = 1993ApJ...413L..67R |url = http://articles.adsabs.harvard.edu/cgi-bin/nph-iarticle_query?1993ApJ...413L..67R&data_type=PDF_HIGH&whole_paper=YES&type=PRINTER&filetype=.pdf }}</ref> | |||
| ===Pyrolysis and combustion product=== | |||
| Certain ], the ], contain cyanide ]s attached to iron in their active sites. The biosynthesis of cyanide in the -hydrogenases proceeds from ]phosphate, which converts to ]yl ], the CN<sup>−</sup> donor.<ref>{{cite journal |last=Reissmann |first=Stefanie |coauthors=Elisabeth Hochleitner, Haofan Wang, Athanasios Paschos, Friedrich Lottspeich, Richard S. Glass and August Böck |title=Taming of a Poison: Biosynthesis of the NiFe-Hydrogenase Cyanide Ligands |url=http://www.sciencemag.org/cgi/content/abstract/299/5609/1067 |accessdate=2008-06-28 |journal=] |year=2003 |volume=299 |issue=5609 |pages=1067–1070 |doi=10.1126/science.1080972 |pmid=12586941}}</ref> | |||
| Hydrogen cyanide is produced by the combustion or ] of certain materials under oxygen-deficient conditions. For example, it can be detected in the ] of ]s and ] smoke. Certain ]s, especially those derived from ], release hydrogen cyanide when heated or burnt.<ref name="CDC"/> | |||
| ===Organic derivatives=== | ===Organic derivatives=== | ||
| {{ |
{{Main|Nitriles}} | ||
| {{see also|Isocyanide}} | |||
| Because of the cyanide anion's high ], a cyano group is readily introduced into organic molecules by displacement of a ] group (e.g. the ] on ]). Organic cyanides are generally called nitriles. Thus, CH<sub>3</sub>CN can be methyl cyanide but more commonly is referred to as ]. In organic synthesis, cyanide is used as a C-1 ]. I.e., it can be used to lengthen a carbon chain by one, while retaining the ability to be functionalized. | |||
| In ], ]s that have a {{chem2|\sC\tN}} ] are called ]s.<ref>] </ref><ref>NCBI-MeSH </ref> An example of a nitrile is ], {{chem2|CH3\sC\tN}}. Nitriles usually do not release cyanide ions. A functional group with a hydroxyl {{chem2|\sOH}} and cyanide {{chem2|\sCN}} bonded to the same carbon atom is called ] ({{chem2|R2C(OH)CN}}). Unlike nitriles, cyanohydrins do release poisonous ]. | |||
| :RX + CN<sup>−</sup> → RCN + X<sup>−</sup> (]) followed by | |||
| # RCN + 2 H<sub>2</sub>O → ] + NH<sub>3</sub> (] under reflux with mineral acid catalyst), or | |||
| # RCN + ] + (second step) 2 H<sub>2</sub>O → ] + 0.5 LiAl(OH)<sub>4</sub> (under ] in dry ], followed by addition of H<sub>2</sub>O) | |||
| == |
==Reactions== | ||
| === |
===Protonation=== | ||
| Cyanide is basic. The p''K''<sub>a</sub> of hydrogen cyanide is 9.21. Thus, addition of ] stronger than hydrogen cyanide to solutions of cyanide salts releases ]. | |||
| {{main|Gold cyanidation}} | |||
| Cyanide is mainly produced for the mining of ] and ]: it helps dissolve these metals and their ores. In the so-called '']'', finely ground high-grade ore is mixed with the cyanide (concentration of about two kilogram NaCN per tonne); low-grade ores are stacked into heaps and sprayed with a cyanide solution (concentration of about one kilogram NaCN per ton). The precious metals are complexed by the cyanide ]s to form soluble derivatives, e.g. <sup>−</sup> and <sup>−</sup>.<ref name=Ullmann>Andreas Rubo, Raf Kellens, Jay Reddy, Norbert Steier, Wolfgang Hasenpusch "Alkali Metal Cyanides" in Ullmann's Encyclopedia of Industrial Chemistry 2006 Wiley-VCH, Weinheim, Germany.ISBN 10.1002/14356007.i01 i01</ref> | |||
| ::2 Au + 4 KCN + ½ O<sub>2</sub> + H<sub>2</sub>O → 2 K + 2 KOH | |||
| ===Hydrolysis=== | |||
| Silver is less "noble" than gold and often occurs as the sulfide, in which case redox is not invoked (no O<sub>2</sub> is required), instead a displacement reaction occurs: | |||
| Cyanide is unstable in water, but the reaction is slow until about 170 °C. It undergoes ] to give ] and ], which are far less toxic than cyanide:<ref name=Ullmann/> | |||
| ::Ag<sub>2</sub>S + 4 KCN → 2 K + K<sub>2</sub>S | |||
| :{{chem2|CN- + 2 H2O → HCO2- + NH3}} | |||
| The "pregnant liquor" containing these ions is separated from the solids, which are discarded to a tailing pond or spent heap, the recoverable gold having been removed. The metal is recovered from the "pregnant solution" by reduction with ] dust or by adsorption onto activated carbon. This process can result in environmental and health problems. Aqueous cyanide is hydrolyzed rapidly, especially in sunlight. It can mobilize some heavy metals such as mercury if present. Gold can also be associated with arsenopyrite (FeAsS), which is similar to ] (fool's gold), wherein half of the sulfur atoms are replaced by ]. Gold-containing arsenopyrite ores are similarly reactive toward inorganic cyanide. | |||
| ] is an ] that catalyzes this reaction. | |||
| ===Alkylation=== | |||
| Cyanide is also used in ]. | |||
| Because of the cyanide anion's high ], cyano groups are readily introduced into organic molecules by displacement of a ] group (e.g., the ] on ]). In general, organic cyanides are called nitriles. In organic synthesis, cyanide is a C-1 ]; i.e., it can be used to lengthen a carbon chain by one, while retaining the ability to be ].<ref>{{Ullmann|doi=10.1002/14356007.a17_363|title=Nitriles|year=2000|last1=Pollak|first1=Peter|last2=Romeder|first2=Gérard|last3=Hagedorn|first3=Ferdinand|last4=Gelbke|first4=Heinz-Peter|isbn=3-527-30673-0}}</ref> | |||
| :{{chem2|RX + CN- → RCN + X-}} | |||
| ===Redox=== | |||
| ===Industrial organic chemistry=== | |||
| The cyanide ion is a ] and is ] by strong ]s such as molecular ] ({{chem2|Cl2}}), ] ({{chem2|ClO-}}), and ] ({{chem2|H2O2}}). These oxidizers are used to destroy cyanides in ]s from ].<ref name="Young_1995">Young, C. A., & Jordan, T. S. (1995, May). Cyanide remediation: current and past technologies. In: Proceedings of the 10th Annual Conference on Hazardous Waste Research (pp. 104–129). Kansas State University: Manhattan, KS. https://engg.ksu.edu/HSRC/95Proceed/young.pdf</ref><ref name="SRK">{{Cite web |title=Cyanide Destruction {{!}} SRK Consulting |author=Dmitry Yermakov |work=srk.com |date= |access-date=2 March 2021 |url= https://www.srk.com/en/publications/cyanide-destruction |language=English}}</ref><ref name="Botz">Botz Michael M. Overview of cyanide treatment methods. Elbow Creek Engineering, Inc. http://www.botz.com/MEMCyanideTreatment.pdf</ref> | |||
| Some nitriles are produced on a large scale, e.g. adiponitrile is a precursor to ]. Such compounds are often generated by combining hydrogen cyanide and alkenes, i.e., ]: | |||
| RCH=CH<sub>2</sub> + HCN → RCH(CN)CH<sub>3</sub> | |||
| Metal catalysts are required for such reactions. | |||
| === |
===Metal complexation=== | ||
| The cyanide anion reacts with ] to form ]. This reaction is the basis of cyanide's toxicity.<ref>Sharpe, A. G. The Chemistry of Cyano Complexes of the Transition Metals; Academic Press: London, 1976{{page needed|date=July 2015}}</ref> The high affinities of metals for this ] can be attributed to its negative charge, compactness, and ability to engage in π-bonding. | |||
| The cyanide compound ] is occasionally used in emergency medical situations to produce a rapid decrease in ] in humans; it is also used as a ] in vascular research. The cobalt in artificial ] contains a cyanide ligand as an artifact of the purification process. During ], a copper cyanide compound was briefly used by ]ese physicians for the treatment of ] and ].<ref>{{cite journal |last=Takano |first=R. |year=1916 |month=August |title=The treatment of leprosy with cyanocuprol |journal=The Journal of Experimental Medicine |volume=24 |issue= |pages=207–211 |url=http://www.jem.org/cgi/content/abstract/24/2/207 |accessdate=2008-06-28 |doi=10.1084/jem.24.2.207 |pmc=2125457}}</ref> | |||
| Among the most important cyanide coordination compounds are the ] and the pigment ], which are both essentially nontoxic due to the tight binding of the cyanides to a central iron atom.<ref name=Holl>{{ cite book |author1=Holleman, A. F. |author2=Wiberg, E. | title = Inorganic Chemistry | publisher = Academic Press | location = San Diego | year = 2001 | isbn = 978-0-12-352651-9 }}</ref> | |||
| ===Fishing=== | |||
| Prussian blue was first accidentally made around 1706, by heating substances containing iron and carbon and nitrogen, and other cyanides made subsequently (and named after it). Among its many uses, Prussian blue gives the blue color to ], ], and ]s. | |||
| {{main|Cyanide fishing}} | |||
| Cyanides are illegally used to capture live fish near ]s for the ] and seafood markets. The practice is controversial, dangerous, and damaging but is driven by the lucrative western market for tropical fish. | |||
| == |
==Manufacture== | ||
| {{main|Hydrogen cyanide#Production and synthesis}} | |||
| ] is used to achieve a blue color on cast ]s during the final finishing stage of the sculpture. On its own, it will produce a very dark shade of blue and is often mixed with other chemicals to achieve the desired tint and hue. It is applied using a torch and paint brush while wearing the standard safety equipment used for any patina application: rubber gloves, safety glasses, and a respirator. The actual amount of cyanide in the mixture varies according to the recipes used by each foundry. | |||
| The principal process used to manufacture cyanides is the ] in which gaseous ] is produced from ] and ] in the presence of ] and a ] ].<ref>{{cite journal | |||
| |title=Über die schnell verlaufenden katalytischen Prozesse in strömenden Gasen und die Ammoniak-Oxydation (V) |trans-title=About the quicka catalytic processes in flowing gases and the ammonia oxidation (V) |language=de |author-link1=Leonid Andrussow |first1=Leonid |last1=Andrussow |journal=Berichte der Deutschen Chemischen Gesellschaft |volume=60 |issue=8 |pages=2005–2018 |year=1927 |doi=10.1002/cber.19270600857 }}</ref><ref>{{cite journal |title=Über die katalytische Oxydation von Ammoniak-Methan-Gemischen zu Blausäure |trans-title=About the catalytic oxidation of ammonia-methane mixtures to cyanide |language=de |first1=L. |last1=Andrussow |journal=] |volume=48 |issue=37 |pages=593–595 |year=1935 |doi=10.1002/ange.19350483702 |bibcode=1935AngCh..48..593A }}</ref> | |||
| :{{chem2|2 CH4 + 2 NH3 + 3 O2 → 2 HCN + 6 H2O}} | |||
| Cyanide is also used in ]-making and certain kinds of ]. | |||
| Sodium cyanide, the precursor to most cyanides, is produced by treating ] with ]:<ref name=Ullmann /> | |||
| Cyanides are used as ]s for fumigating ships. Cyanide salts are used for killing ants, and have in some places been used as rat poison (the less toxic poison ] is more common{{Citation needed|date=October 2008}}). | |||
| :{{chem2|HCN + NaOH → NaCN + H2O}} | |||
| ==Toxicity== | |||
| ==Chemical tests for cyanide== | |||
| {{Main|Cyanide poisoning}} | |||
| ===Prussian blue=== | |||
| Among the most toxic cyanides are hydrogen cyanide ({{chem|HCN}}), sodium cyanide ({{chem|NaCN}}), potassium cyanide ({{chem|KCN}}), and calcium cyanide ({{chem2|Ca(CN)2}}). The cyanide anion is an ] of the ] ] (also known as aa<sub>3</sub>), the fourth complex of the ] found in the ] of the ] of ] cells. It attaches to the iron within this protein. The binding of cyanide to this enzyme prevents transport of electrons from ] to oxygen. As a result, the electron transport chain is disrupted, meaning that the cell can no longer aerobically produce ] for energy.<ref>{{cite book|last1=Nelson|first1=David L.|last2=Cox|first2=Michael M.|title=Lehniger Principles of Biochemistry|publisher=]|year=2000|location=New York|edition=3rd|isbn=978-1-57259-153-0|pages=|url=https://archive.org/details/lehningerprincip01lehn/page/668}}</ref> Tissues that depend highly on ], such as the ] and the ], are particularly affected. This is an example of ].<ref name=Biller>{{cite book | |||
| ] is added to a solution suspected of containing cyanide, such as the filtrate from the ]. The resulting mixture is acidified with ]. The formation of ] is a positive result for cyanide. | |||
| |title=Interface of neurology and internal medicine | |||
| |edition=illustrated | |||
| |first1=José | |||
| |last1=Biller | |||
| |publisher=Lippincott Williams & Wilkins | |||
| |year=2007 | |||
| |isbn=978-0-7817-7906-7 | |||
| |chapter=163 | |||
| |page=939 | |||
| |chapter-url=https://books.google.com/books?id=SRIvmTVcYBwC&pg=PA939}} | |||
| </ref> | |||
| The most hazardous compound is ], which is a gas and kills by inhalation. For this reason, working with hydrogen cyanide requires wearing an air respirator supplied by an external oxygen source.<ref name="CDC">{{Cite web|url=https://emergency.cdc.gov/agent/cyanide/basics/facts.asp|title=Facts about cyanide:Where cyanide is found and how it is used|last=Anon|date=June 27, 2013|work=CDC Emergency preparedness and response|publisher=Centers for Disease Control and Prevention|access-date=10 December 2016}}</ref> Hydrogen cyanide is produced by adding acid to a solution containing a cyanide salt. Alkaline solutions of cyanide are safer to use because they do not evolve hydrogen cyanide gas. Hydrogen cyanide may be produced in the combustion of ]s; for this reason, polyurethanes are not recommended for use in domestic and aircraft furniture. Oral ingestion of a small quantity of solid cyanide or a cyanide solution of as little as 200 mg, or exposure to airborne cyanide of 270 ], is sufficient to cause death within minutes.<ref name=Biller/> | |||
| ===''para''-Benzoquinone in DMSO=== | |||
| A solution of ''para''-] in ] reacts with inorganic cyanide to form a cyano], which is ]. Illumination with a ] gives a green/blue glow if the test is positive.<ref>{{cite journal | doi = 10.1016/0041-008X(80)90225-2 }}</ref> | |||
| Organic ]s do not readily release cyanide ions, and so have low toxicities. By contrast, compounds such as ] {{chem2|(CH3)3SiCN}} readily release HCN or the cyanide ion upon contact with water.<ref>{{cite web |url=https://www.gelest.com/wp-content/uploads/product_msds/SIT8585.1-msds.pdf |archive-url=https://ghostarchive.org/archive/20221010/https://www.gelest.com/wp-content/uploads/product_msds/SIT8585.1-msds.pdf |archive-date=2022-10-10 |url-status=live |title=MSDS of trimethylsilyl cyanide |publisher=Gelest Inc |date=2008 |access-date=2022-08-16}}</ref> | |||
| ===Copper and an aromatic amine=== | |||
| As used by ] to detect ], ](II) salt and an aromatic amine such as ] is added to the sample; as an alternative to benzidine an alternative amine di-(4,4-''bis''-dimethylaminophenyl) methane can be used. A positive test gives a blue color. ] is poorly soluble. By ] the copper(I) the copper(II) is rendered a stronger ]. The copper, in a cyanide facilitated oxidation, converts the ] into a colored compound. The ] explains this process. Another good example of such chemistry is the way in which the saturated ] ] (]) works. The copper, in a cyanide facilitated oxidation converts the ] into a colored compound. | |||
| ===Antidote=== | |||
| ===Pyridine-barbituric acid colorimetry=== | |||
| ] reacts with cyanide to form ], which can be safely eliminated by the kidneys. This method has the advantage of avoiding the formation of methemoglobin (see below). This antidote kit is sold under the brand name Cyanokit and was approved by the U.S. FDA in 2006.<ref>{{EMedicine|article|814287|Cyanide Toxicity|treatment}}</ref> | |||
| A sample containing inorganic cyanide is purged with air from a boiling acid solution into a basic absorber solution. The cyanide salt absorbed in the basic solution is buffered at pH 4.5 and then reacted with chlorine to form cyanogen chloride. The cyanogen chloride formed couples pyridine with barbituric acid to form a strongly colored red dye that is proportional to the cyanide concentration. This colorimetric method following distillation is the basis for most regulatory methods (for instance EPA 335.4) used to analyze cyanide in water, wastewater, and contaminated soils. Distillation followed by colorimetric methods, however, have been found to be prone to interferences from thiocyanate, nitrate, thiosulfate, sulfite, and sulfide that can result in both positive and negative bias. It has been recommended by the USEPA (MUR March 12, 2007) that samples containing these compounds be analyzed by Gas-Diffusion Flow Injection Analysis — Amperometry.{{Citation needed|date=June 2008}} | |||
| An older cyanide antidote kit included administration of three substances: ] pearls (administered by inhalation), ], and ]. The goal of the antidote was to generate a large pool of ] iron ({{chem2|Fe(3+)}}) to compete for cyanide with cytochrome a<sub>3</sub> (so that cyanide will bind to the antidote rather than the enzyme). The ]s ] ] to ], which competes with cytochrome oxidase for the cyanide ion. Cyanmethemoglobin is formed and the ] enzyme is restored. The major mechanism to remove the cyanide from the body is by enzymatic conversion to ] by the ] enzyme ]. Thiocyanate is a relatively non-toxic molecule and is excreted by the kidneys. To accelerate this detoxification, sodium thiosulfate is administered to provide a sulfur donor for ], needed in order to produce thiocyanate.<ref>{{cite journal | last1 = Chaudhary | first1 = M. | last2 = Gupta | first2 = R. | year = 2012 | title = Cyanide Detoxifying Enzyme: Rhodanese | journal = Current Biotechnology | volume = 1 | issue = 4 | pages = 327–335 | doi = 10.2174/2211550111201040327 }}</ref> | |||
| ===Gas diffusion flow injection analysis — amperometry=== | |||
| Instead of distilling, the sample is injected into an acidic stream where the HCN formed is passed under a hydrophobic gas diffusion membrane that selectively allows only HCN to pass through. The HCN that passes through the membrane is absorbed into a basic carrier solution that transports the CN to an amperometric detector that accurately measures cyanide concentration with high sensitivity. Sample pretreatment determined by acid reagents, ligands, or preliminary UV irradiation allow cyanide speciation of free cyanide, available cyanide, and total cyanide respectively. These relative simplicity of these flow injection analysis methods limit the interference experienced by the high heat of distillation and also prove to be cost effective since time consuming distillations are not required. | |||
| == |
===Sensitivity=== | ||
| Minimum risk levels (MRLs) may not protect for delayed health effects or health effects acquired following repeated sublethal exposure, such as hypersensitivity, ], or ]. MRLs may be revised after sufficient data accumulates.<ref>{{cite report|title=Toxicological Profile for Cyanide |publisher=U.S. Department of Health and Human Services |date=2006 |url=https://www.atsdr.cdc.gov/toxprofiles/tp8.pdf |archive-url=https://web.archive.org/web/20040331014808/http://www.atsdr.cdc.gov/toxprofiles/tp8.pdf |archive-date=2004-03-31 |url-status=live |pages=18–19}}</ref> | |||
| {{main|Cyanide poisoning}} | |||
| Many cyanide-containing compounds are highly toxic, but some are not. ]s (which do not release cyanide ions) and hexacyanoferrates (] and ], where the cyanide is already tightly bound to an ] ion) have low toxicities, while most other cyanides are deadly poisonous. ], with an approximate formula Fe<sub>7</sub>(CN)<sub>18</sub> is the blue of ]s and is administered orally as an antidote to poisoning by ] and radioactive ]; the large ferrocyanide anion is an effective getter for heavy monovalent cations. The most dangerous cyanides are ] (HCN) and salts derived from it, such as potassium cyanide (KCN) and sodium cyanide (NaCN), among others. Also some compounds readily release HCN or the cyanide ion, such as ] (CH<sub>3</sub>)<sub>3</sub>SiCN upon contact with water and ]s upon ]. {{Citation needed|date=February 2007}} | |||
| ==Applications== | |||
| The cyanide anion is an ] of the ] ] (also known as aa<sub>3</sub>) in the fourth complex of the ] (found in the membrane of the ] of eukaryotic cells). It attaches to the iron within this protein. The binding of cyanide to this cytochrome prevents transport of electrons from ] to oxygen. As a result, the electron transport chain is disrupted, meaning that the cell can no longer aerobically produce ] for energy. Tissues that mainly depend on ], such as the ] and the ], are particularly affected. | |||
| === |
===Mining=== | ||
| {{Main|Gold cyanidation}} | |||
| ] reacts with cyanide to form ]. Cyanocobalamin can be eliminated by the kidneys. This method has the advantage of avoiding the formation of methemoglobin (see below). This antidote kit is sold under the brand name Cyanokit and was approved by the FDA in 2006. <ref>http://emedicine.medscape.com/article/814287-treatment</ref> | |||
| Cyanide is mainly produced for the ] of ] and ]: It helps dissolve these metals allowing separation from the other solids. In the '']'', finely ground high-grade ore is mixed with the cyanide (at a ratio of about 1:500 parts NaCN to ore); low-grade ores are stacked into heaps and sprayed with a cyanide solution (at a ratio of about 1:1000 parts NaCN to ore). The precious metals are complexed by the cyanide ]s to form soluble derivatives, e.g., {{chem2|-}} (dicyanoargentate(I)) and {{chem2|-}} (dicyanoaurate(I)).<ref name=Ullmann>{{Ullmann |first1=Andreas |last1=Rubo |first2=Raf |last2=Kellens |first3=Jay |last3=Reddy |first4=Norbert |last4=Steier |first5=Wolfgang |last5=Hasenpusch |title=Alkali Metal Cyanides |year=2006 |doi=10.1002/14356007.i01_i01}}</ref> Silver is less ] than gold and often occurs as the sulfide, in which case redox is not invoked (no {{chem2|O2}} is required). Instead, a displacement reaction occurs: | |||
| :<chem>Ag2S + 4 NaCN + H2O -> 2 Na + NaSH + NaOH</chem> | |||
| :<chem>4 Au + 8 NaCN + O2 + 2 H2O -> 4 Na + 4 NaOH</chem> | |||
| The "pregnant liquor" containing these ions is separated from the solids, which are discarded to a ] or spent heap, the recoverable gold having been removed. The metal is recovered from the "pregnant solution" by reduction with ] dust or by ] onto ]. This process can result in environmental and health problems. A number of ] have followed the overflow of tailing ponds at gold mines. Cyanide contamination of waterways has resulted in numerous cases of human and aquatic species mortality.<ref>{{cite journal |last1=Kumar |first1=Rahul |last2=Saha |first2=Shouvik |last3=Sarita |first3=Dhaka |last4=Mayur B. |first4=Kurade |last5=Kang |first5=Chan Ung |last6=Baek |first6=Seung Han |last7=Jeong |first7=Byong-Hun |title=Remediation of cyanide-contaminated environments through microbes and plants: a review of current knowledge and future perspectives |journal=Geosystem Engineering |date=2016 |volume=70 |issue=1 |pages=28–40 |doi=10.1080/12269328.2016.1218303 |s2cid=132571397 |url=https://www.tandfonline.com/doi/full/10.1080/12269328.2016.1218303 |access-date=24 April 2022}}</ref> | |||
| Aqueous cyanide is hydrolyzed rapidly, especially in sunlight. It can mobilize some heavy metals such as mercury if present. Gold can also be associated with arsenopyrite (FeAsS), which is similar to ] (fool's gold), wherein half of the sulfur atoms are replaced by ]. Gold-containing arsenopyrite ores are similarly reactive toward inorganic cyanide.<ref>{{Cite journal |last=Konyratbekova |first=Saltanat Sabitovna |last2=Baikonurova |first2=Aliya |last3=Akcil |first3=Ata |date=2015-05-04 |title=Non-cyanide Leaching Processes in Gold Hydrometallurgy and Iodine-Iodide Applications: A Review |url=http://www.tandfonline.com/doi/abs/10.1080/08827508.2014.942813 |journal=Mineral Processing and Extractive Metallurgy Review |language=en |volume=36 |issue=3 |pages=198–212 |doi=10.1080/08827508.2014.942813 |issn=0882-7508}}</ref><ref>{{Cite journal |last=Zhang |first=Yan |last2=Cui |first2=Mingyao |last3=Wang |first3=Jianguo |last4=Liu |first4=Xiaoliang |last5=Lyu |first5=Xianjun |date=2022 |title=A review of gold extraction using alternatives to cyanide: Focus on current status and future prospects of the novel eco-friendly synthetic gold lixiviants |url=https://linkinghub.elsevier.com/retrieve/pii/S0892687521005653 |journal=Minerals Engineering |language=en |volume=176 |pages=107336 |doi=10.1016/j.mineng.2021.107336}}</ref> | |||
| An older cyanide antidote kit included administration of three substances: amyl nitrite pearls (inhalation) and sodium nitrite and sodium thiosulfate (infusion). The goal of the antidote is to generate a large pool of ferric iron to compete with cytochrome a<sub>3</sub> (part of the electron transport chain necessary for cellular respiration/energy production) for cyanide. The ]s ] ] to ] which competes with cytochrome oxidase for the cyanide ion. Cyanmethemoglobin is formed and ] is restored. The major mechanism to remove the cyanide from the body is by enzymatic conversion by the ] enzyme ] to convert cyanate to ], which is a relatively non-toxic molecule that is excreted in the urine. To accelerate the detoxification sodium thiosulfate is administered to provide a sulfur donor for rhodanese to produce thiocyanate. | |||
| ===Industrial organic chemistry=== | |||
| ===Sensitivity=== | |||
| The second major application of alkali metal cyanides (after mining) is in the production of CN-containing compounds, usually nitriles. ]s are produced from acyl chlorides and cyanide. ], ], and the trimer ] are derived from alkali metal cyanides. | |||
| Minimum Risk Levels (MRLs) may not protect for delayed health effects or health effects acquired following repeated sublethal exposure, like hypersensitivity, asthma, or bronchitis. MRLs may be revised after sufficient data accumulates (Toxicological Profile for Cyanide, U.S. Department of Health and Human Services, 2006). | |||
| ===Medical uses=== | |||
| == Notable cyanide deaths == | |||
| The cyanide compound ] is used mainly in ] to measure ] ] mainly as a follow-up to ] patients. On occasion, it is used in emergency medical situations to produce a rapid decrease in ] in humans; it is also used as a ] in vascular research. The cobalt in artificial ] contains a cyanide ligand as an artifact of the purification process; this must be removed by the body before the vitamin molecule can be activated for biochemical use. During ], a copper cyanide compound was briefly used by ]ese physicians for the treatment of ] and ].<ref>{{Cite journal|last=Takano |first=R. |date=August 1916 |title=The treatment of leprosy with cyanocuprol |journal=The Journal of Experimental Medicine |volume=24 |issue= 2|pages=207–211 |url=http://www.jem.org/cgi/content/abstract/24/2/207 |access-date=2008-06-28 |doi=10.1084/jem.24.2.207 |pmc=2125457 |pmid=19868035}}</ref> | |||
| Cyanides have been used as poison many times throughout history. The most infamous application was the use of ] (in ] pellets) by the ] regime in Germany for mass murder in some ]s during ]. Cyanides have been used (as in the case of ]) for attempted murder, and for judicial execution in some parts of the United States. Some notable persons who committed suicide by cyanides (either cyanide salt or hydrogen cyanide) are ], ], ], ], ], ], ] (in combination with a gunshot), ], ], ] and the ]. The mass suicide/murder of The People's Temple in ] was done with cyanide poisoning. | |||
| ===Illegal fishing and poaching=== | |||
| ==References== | |||
| {{Main|Cyanide fishing}} | |||
| {{reflist|2}} | |||
| Cyanides are illegally used to capture live fish near ]s for the ] and seafood markets. The practice is controversial, dangerous, and damaging but is driven by the lucrative exotic fish market.<ref name="crc">Dzombak, David A; Ghosh, Rajat S; Wong-Chong, George M. ''Cyanide in Water and Soil''. ], 2006, Chapter 11.2: "Use of Cyanide for Capturing Live Reef Fish".</ref> | |||
| Poachers in Africa have been known to use cyanide to poison waterholes, to kill elephants for their ivory.<ref> ''ABC News'', 25 September 2013. Retrieved 30 October 2015.</ref> | |||
| ==Sources== | |||
| ===Pest control=== | |||
| *Institut national de recherche et de sécurité (1997). "". ''Fiche toxicologique n° 4'', Paris:INRS, 5pp. (PDF file, ''in French'') | |||
| ] are used in the United States to kill ]s and other canids.<ref>{{cite journal|doi=10.1002/wsb.361|title=Animal attendance at M-44 sodium cyanide ejector sites for coyotes|journal=Wildlife Society Bulletin|volume=38|pages=217–220|year=2014|last1=Shivik|first1=John A.|last2=Mastro|first2=Lauren|last3=Young|first3=Julie K. |issue=1 |bibcode=2014WSBu...38..217S |url=http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=2419&context=icwdm_usdanwrc}}</ref> Cyanide is also used for pest control in ], particularly for ], an ] and spreads ] amongst cattle. Possums can become bait shy but the use of pellets containing the cyanide reduces bait shyness. Cyanide has been known to kill native birds, including the endangered ].<ref>{{cite web |last = Green| first = Wren |title =The use of 1080 for pest control |publisher = New Zealand Department of Conservation |date = July 2004 |url =http://www.doc.govt.nz/upload/documents/conservation/threats-and-impacts/animal-pests/use-of-1080-04.pdf| access-date = 8 June 2011}}</ref> Cyanide is also effective for controlling the ], another introduced marsupial pest in New Zealand.<ref>{{cite journal|last=Shapiro|first=Lee|date=21 March 2011|title=Effectiveness of cyanide pellets for control of dama wallabies (Macropus eugenii)|journal=New Zealand Journal of Ecology |volume=35 |issue=3 |url=http://newzealandecology.org/nzje/new_issues/NZJEcol35_3_287.pdf |archive-url=https://web.archive.org/web/20150203010818/http://newzealandecology.org/nzje/new_issues/NZJEcol35_3_287.pdf |archive-date=2015-02-03 |url-status=live |display-authors=etal}}</ref> A licence is required to store, handle and use cyanide in New Zealand. | |||
| *Institut national de recherche et de sécurité (1997). "". ''Fiche toxicologique n° 111'', Paris:INRS, 6pp. (PDF file, ''in French'') | |||
| Cyanides are used as ]s for fumigating ships.<ref>{{cite web|title=Sodium Cyanide|url=https://pubchem.ncbi.nlm.nih.gov/compound/sodium_cyanide|website=PubChem|publisher=National Center for Biotechnology Information|access-date=2 September 2016|date=2016|quote=Cyanide and hydrogen cyanide are used in electroplating, metallurgy, organic chemicals production, photographic developing, manufacture of plastics, fumigation of ships, and some mining processes.}}</ref> Cyanide salts are used for killing ants,<ref name="EPAReg1994">{{cite web|title=Reregistration Eligibility Decision (RED) Sodium Cyanide|url=https://archive.epa.gov/pesticides/reregistration/web/pdf/3086.pdf |archive-url=https://ghostarchive.org/archive/20221010/https://archive.epa.gov/pesticides/reregistration/web/pdf/3086.pdf |archive-date=2022-10-10 |url-status=live|website=EPA.gov|access-date=2 September 2016|page=7|date=1 September 1994|quote=Sodium cyanide was initially registered as a pesticide on December 23, 1947, to control ants on uncultivated agricultural and non-agricultural areas.}}</ref> and have in some places been used as rat poison<ref name="TariffInfo1921">{{cite web|title=Tariff Information, 1921: Hearings on General Tariff Revision Before the Committee on Ways and Means, House of Representatives|url=http://www.abebooks.com/servlet/SearchResults?tn=Tariff+Information,+1921|website=AbeBooks.com|publisher=US Congress, House Committee on Ways and Means, US Government Printing Office|access-date=2 September 2016|page=3987|date=1921|quote=Another field in which cyanide is used in growing quantity is the eradication of rats and other vermin – especially in the fight against typhus.}}</ref> (the less toxic poison ] is more common).<ref name="PlanetDeadly2013">{{cite web|title=Deadliest Poisons Used by Man|url=http://www.planetdeadly.com/human/deadliest-poisons-man|website=PlanetDeadly.com|access-date=2 September 2016|archive-url=https://web.archive.org/web/20160511033535/http://www.planetdeadly.com/human/deadliest-poisons-man|archive-date=11 May 2016|date=18 November 2013}}</ref> | |||
| ===Niche uses=== | |||
| ] is used to achieve a blue color on cast ]s during the final finishing stage of the sculpture. On its own, it will produce a very dark shade of blue and is often mixed with other chemicals to achieve the desired tint and hue. It is applied using a torch and paint brush while wearing the standard safety equipment used for any patina application: rubber gloves, safety glasses, and a respirator. The actual amount of cyanide in the mixture varies according to the recipes used by each foundry. | |||
| Cyanide is also used in ]-making and certain kinds of ] such as ]. | |||
| Although usually thought to be toxic, cyanide and cyanohydrins increase germination in various plant species.<ref>{{Cite journal|doi=10.1104/pp.52.1.23 |last1=Taylorson |first1=R. |last2=Hendricks |year=1973 |first2=SB |title=Promotion of Seed Germination by Cyanide |journal=Plant Physiol. |volume=52 |issue=1 |pages=23–27 |pmid=16658492 |pmc=366431}}</ref><ref>{{Cite journal|last1=Mullick |first1=P. |year=1967 |last2=Chatterji |first2=U. N. |title=Effect of sodium cyanide on germination of two leguminous seeds |journal=Plant Systematics and Evolution |volume=114 |pages=88–91|doi=10.1007/BF01373937|s2cid=2533762 }}</ref> | |||
| ====Human poisoning==== | |||
| {{main|Cyanide poisoning}} | |||
| Deliberate cyanide poisoning of humans has occurred many times throughout history.<ref>{{Cite book | |||
| |title=Medical Management of Chemical Casualties Handbook | |||
| |edition=4th | |||
| |last1=Bernan | |||
| |publisher=Government Printing Off | |||
| |year=2008 | |||
| |isbn=978-0-16-081320-7 | |||
| |page=41 | |||
| |url=https://books.google.com/books?id=oiw2ZzsBvsoC}}, | |||
| </ref> | |||
| Common salts such as ] are involatile but water-soluble, so are poisonous by ingestion. ] is a gas, making it more indiscriminately dangerous, however it is lighter than air and rapidly disperses up into the atmosphere, which makes it ineffective as a ]. | |||
| ====Food additive==== | |||
| Because of the high stability of their complexation with ], ferrocyanides (] E535, ] E536, and Calcium ferrocyanide E538<ref>{{cite book | |||
| |title=Benders' dictionary of nutrition and food technology | |||
| |edition=7th | |||
| |first1=David A. | |||
| |last1=Bender | |||
| |first2=Arnold Eric | |||
| |last2=Bender | |||
| |publisher=Woodhead Publishing | |||
| |year=1997 | |||
| |isbn=978-1-85573-475-3 | |||
| |page=459 | |||
| |url=https://books.google.com/books?id=IrYfDEl7XPYC}} | |||
| </ref>) do not decompose to lethal levels in the human body and are used in the food industry as, e.g., an ] in ].<ref>{{cite book | |||
| |title=Geochemical processes in soil and groundwater: measurement – modelling – upscaling | |||
| |first1=Horst D. | |||
| |last1=Schulz | |||
| |first2=Astrid | |||
| |last2=Hadeler | |||
| |author3=Deutsche Forschungsgemeinschaft | |||
| |publisher=Wiley-VCH | |||
| |year=2003 | |||
| |isbn=978-3-527-27766-7 | |||
| |page=67 | |||
| |doi=10.1002/9783527609703 | |||
| |url=http://onlinelibrary.wiley.com/doi/10.1002/9783527609703}} | |||
| </ref> | |||
| ==Chemical tests for cyanide== | |||
| Cyanide is quantified by ], a method widely used in gold mining. It can also be determined by titration with silver ion. | |||
| Some analyses begin with an air-purge of an acidified boiling solution, sweeping the vapors into a basic absorber solution. The cyanide salt absorbed in the basic solution is then analyzed.<ref>{{Ullmann|doi=10.1002/14356007.a08_159.pub2|title=Cyano Compounds, Inorganic|year=2004|last1=Gail|first1=Ernst|last2=Gos|first2=Stephen|last3=Kulzer|first3=Rupprecht|last4=Lorösch|first4=Jürgen|last5=Rubo|first5=Andreas|last6=Sauer|first6=Manfred}}</ref> | |||
| ===Qualitative tests=== | |||
| Because of the notorious toxicity of cyanide, many methods have been investigated. Benzidine gives a blue coloration in the presence of ].<ref>{{Ullmann |author1=Schwenecke, H. |author2=Mayer, D. | title = Benzidine and Benzidine Derivatives | year = 2005 | doi = 10.1002/14356007.a03_539 }}</ref> ] added to a solution of cyanide, such as the filtrate from the ], gives ]. A solution of ] in ] reacts with inorganic cyanide to form a cyano], which is ]. Illumination with a ] gives a green/blue glow if the test is positive.<ref>{{Cite journal| doi = 10.1016/0041-008X(80)90225-2 |pmid = 7423496 |title = Fluorometric determination of cyanide in biological fluids with p-benzoquinone*1 |first4 = JL |last4 = Way |first3 = RL |last3 = Morgan |first2 = GE |year = 1980 |last2 = Isom |last1 = Ganjeloo |first1 = A |journal = ] |volume = 55 |issue = 1 |pages = 103–107 }}</ref> | |||
| ==References== | |||
| {{Reflist}} | |||
| ==External links== | ==External links== | ||
| {{EB1911 Poster|Cyanide}} | |||
| * | |||
| {{Commons category|Cyanides}} | |||
| * | |||
| * | |||
| * (] 61) | |||
| * |
* | ||
| * (] 61) | |||
| * | |||
| * | |||
| * | |||
| * | |||
| * | |||
| * | |||
| * | |||
| ;Safety data (French) | |||
| * Institut national de recherche et de sécurité (1997). "". ''Fiche toxicologique n° 4'', Paris: INRS, 5 pp. (PDF file, {{in lang|fr}}) | |||
| * Institut national de recherche et de sécurité (1997). "". ''Fiche toxicologique n° 111'', Paris: INRS, 6 pp. (PDF file, {{in lang|fr}}) | |||
| {{Cyanides}} | |||
| {{Inorganic compounds of carbon}} | {{Inorganic compounds of carbon}} | ||
| {{Nitrogen compounds}} | |||
| {{Rodenticides}} | |||
| {{Consumer Food Safety}} | |||
| {{Authority control}} | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 04:27, 24 December 2024
Any molecule with a cyano group (–C≡N) This article is about the class of chemical compounds. For other uses, see Cyanide (disambiguation). Not to be confused with Nitrile.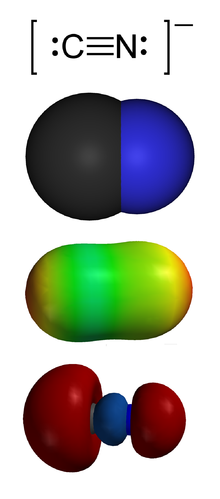
| |
| Names | |
|---|---|
| Systematic IUPAC name Nitridocarbonate(II) | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | CN |
| Molar mass | 26.018 g·mol |
| Conjugate acid | Hydrogen cyanide |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | The cyanide ion CN is one of the most poisonous chemicals. It may cause death in minutes. |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
In chemistry, cyanide (from Greek kyanos 'dark blue') is a chemical compound that contains a C≡N functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
In inorganic cyanides, the cyanide group is present as the cyanide anion C≡N. This anion is extremely poisonous. Soluble salts such as sodium cyanide (NaCN) and potassium cyanide (KCN) are highly toxic. Hydrocyanic acid, also known as hydrogen cyanide, or HCN, is a highly volatile liquid that is produced on a large scale industrially. It is obtained by acidification of cyanide salts.
Organic cyanides are usually called nitriles. In nitriles, the −C≡N group is linked by a single covalent bond to carbon. For example, in acetonitrile (CH3−C≡N), the cyanide group is bonded to methyl (−CH3). Although nitriles generally do not release cyanide ions, the cyanohydrins do and are thus toxic.
Bonding
The cyanide ion C≡N is isoelectronic with carbon monoxide C≡O and with molecular nitrogen N≡N. A triple bond exists between C and N. The negative charge is concentrated on carbon C.
Occurrence
In nature

Cyanides are produced by certain bacteria, fungi, and algae. It is an antifeedant in a number of plants. Cyanides are found in substantial amounts in certain seeds and fruit stones, e.g., those of bitter almonds, apricots, apples, and peaches. Chemical compounds that can release cyanide are known as cyanogenic compounds. In plants, cyanides are usually bound to sugar molecules in the form of cyanogenic glycosides and defend the plant against herbivores. Cassava roots (also called manioc), an important potato-like food grown in tropical countries (and the base from which tapioca is made), also contain cyanogenic glycosides.
The Madagascar bamboo Cathariostachys madagascariensis produces cyanide as a deterrent to grazing. In response, the golden bamboo lemur, which eats the bamboo, has developed a high tolerance to cyanide.
The hydrogenase enzymes contain cyanide ligands attached to iron in their active sites. The biosynthesis of cyanide in the NiFe hydrogenases proceeds from carbamoyl phosphate, which converts to cysteinyl thiocyanate, the CN donor.
Interstellar medium
The cyanide radical CN has been identified in interstellar space. Cyanogen, (CN)2, is used to measure the temperature of interstellar gas clouds.
Pyrolysis and combustion product
Hydrogen cyanide is produced by the combustion or pyrolysis of certain materials under oxygen-deficient conditions. For example, it can be detected in the exhaust of internal combustion engines and tobacco smoke. Certain plastics, especially those derived from acrylonitrile, release hydrogen cyanide when heated or burnt.
Organic derivatives
Main article: Nitriles See also: IsocyanideIn IUPAC nomenclature, organic compounds that have a −C≡N functional group are called nitriles. An example of a nitrile is acetonitrile, CH3−C≡N. Nitriles usually do not release cyanide ions. A functional group with a hydroxyl −OH and cyanide −CN bonded to the same carbon atom is called cyanohydrin (R2C(OH)CN). Unlike nitriles, cyanohydrins do release poisonous hydrogen cyanide.
Reactions
Protonation
Cyanide is basic. The pKa of hydrogen cyanide is 9.21. Thus, addition of acids stronger than hydrogen cyanide to solutions of cyanide salts releases hydrogen cyanide.
Hydrolysis
Cyanide is unstable in water, but the reaction is slow until about 170 °C. It undergoes hydrolysis to give ammonia and formate, which are far less toxic than cyanide:
- CN + 2 H2O → HCO−2 + NH3
Cyanide hydrolase is an enzyme that catalyzes this reaction.
Alkylation
Because of the cyanide anion's high nucleophilicity, cyano groups are readily introduced into organic molecules by displacement of a halide group (e.g., the chloride on methyl chloride). In general, organic cyanides are called nitriles. In organic synthesis, cyanide is a C-1 synthon; i.e., it can be used to lengthen a carbon chain by one, while retaining the ability to be functionalized.
- RX + CN → RCN + X
Redox
The cyanide ion is a reductant and is oxidized by strong oxidizing agents such as molecular chlorine (Cl2), hypochlorite (ClO), and hydrogen peroxide (H2O2). These oxidizers are used to destroy cyanides in effluents from gold mining.
Metal complexation
The cyanide anion reacts with transition metals to form M-CN bonds. This reaction is the basis of cyanide's toxicity. The high affinities of metals for this anion can be attributed to its negative charge, compactness, and ability to engage in π-bonding.
Among the most important cyanide coordination compounds are the potassium ferrocyanide and the pigment Prussian blue, which are both essentially nontoxic due to the tight binding of the cyanides to a central iron atom. Prussian blue was first accidentally made around 1706, by heating substances containing iron and carbon and nitrogen, and other cyanides made subsequently (and named after it). Among its many uses, Prussian blue gives the blue color to blueprints, bluing, and cyanotypes.
Manufacture
Main article: Hydrogen cyanide § Production and synthesisThe principal process used to manufacture cyanides is the Andrussow process in which gaseous hydrogen cyanide is produced from methane and ammonia in the presence of oxygen and a platinum catalyst.
- 2 CH4 + 2 NH3 + 3 O2 → 2 HCN + 6 H2O
Sodium cyanide, the precursor to most cyanides, is produced by treating hydrogen cyanide with sodium hydroxide:
- HCN + NaOH → NaCN + H2O
Toxicity
Main article: Cyanide poisoningAmong the most toxic cyanides are hydrogen cyanide (HCN), sodium cyanide (NaCN), potassium cyanide (KCN), and calcium cyanide (Ca(CN)2). The cyanide anion is an inhibitor of the enzyme cytochrome c oxidase (also known as aa3), the fourth complex of the electron transport chain found in the inner membrane of the mitochondria of eukaryotic cells. It attaches to the iron within this protein. The binding of cyanide to this enzyme prevents transport of electrons from cytochrome c to oxygen. As a result, the electron transport chain is disrupted, meaning that the cell can no longer aerobically produce ATP for energy. Tissues that depend highly on aerobic respiration, such as the central nervous system and the heart, are particularly affected. This is an example of histotoxic hypoxia.
The most hazardous compound is hydrogen cyanide, which is a gas and kills by inhalation. For this reason, working with hydrogen cyanide requires wearing an air respirator supplied by an external oxygen source. Hydrogen cyanide is produced by adding acid to a solution containing a cyanide salt. Alkaline solutions of cyanide are safer to use because they do not evolve hydrogen cyanide gas. Hydrogen cyanide may be produced in the combustion of polyurethanes; for this reason, polyurethanes are not recommended for use in domestic and aircraft furniture. Oral ingestion of a small quantity of solid cyanide or a cyanide solution of as little as 200 mg, or exposure to airborne cyanide of 270 ppm, is sufficient to cause death within minutes.
Organic nitriles do not readily release cyanide ions, and so have low toxicities. By contrast, compounds such as trimethylsilyl cyanide (CH3)3SiCN readily release HCN or the cyanide ion upon contact with water.
Antidote
Hydroxocobalamin reacts with cyanide to form cyanocobalamin, which can be safely eliminated by the kidneys. This method has the advantage of avoiding the formation of methemoglobin (see below). This antidote kit is sold under the brand name Cyanokit and was approved by the U.S. FDA in 2006.
An older cyanide antidote kit included administration of three substances: amyl nitrite pearls (administered by inhalation), sodium nitrite, and sodium thiosulfate. The goal of the antidote was to generate a large pool of ferric iron (Fe) to compete for cyanide with cytochrome a3 (so that cyanide will bind to the antidote rather than the enzyme). The nitrites oxidize hemoglobin to methemoglobin, which competes with cytochrome oxidase for the cyanide ion. Cyanmethemoglobin is formed and the cytochrome oxidase enzyme is restored. The major mechanism to remove the cyanide from the body is by enzymatic conversion to thiocyanate by the mitochondrial enzyme rhodanese. Thiocyanate is a relatively non-toxic molecule and is excreted by the kidneys. To accelerate this detoxification, sodium thiosulfate is administered to provide a sulfur donor for rhodanese, needed in order to produce thiocyanate.
Sensitivity
Minimum risk levels (MRLs) may not protect for delayed health effects or health effects acquired following repeated sublethal exposure, such as hypersensitivity, asthma, or bronchitis. MRLs may be revised after sufficient data accumulates.
Applications
Mining
Main article: Gold cyanidationCyanide is mainly produced for the mining of silver and gold: It helps dissolve these metals allowing separation from the other solids. In the cyanide process, finely ground high-grade ore is mixed with the cyanide (at a ratio of about 1:500 parts NaCN to ore); low-grade ores are stacked into heaps and sprayed with a cyanide solution (at a ratio of about 1:1000 parts NaCN to ore). The precious metals are complexed by the cyanide anions to form soluble derivatives, e.g., [Ag(CN)2] (dicyanoargentate(I)) and [Au(CN)2] (dicyanoaurate(I)). Silver is less "noble" than gold and often occurs as the sulfide, in which case redox is not invoked (no O2 is required). Instead, a displacement reaction occurs:
The "pregnant liquor" containing these ions is separated from the solids, which are discarded to a tailing pond or spent heap, the recoverable gold having been removed. The metal is recovered from the "pregnant solution" by reduction with zinc dust or by adsorption onto activated carbon. This process can result in environmental and health problems. A number of environmental disasters have followed the overflow of tailing ponds at gold mines. Cyanide contamination of waterways has resulted in numerous cases of human and aquatic species mortality.
Aqueous cyanide is hydrolyzed rapidly, especially in sunlight. It can mobilize some heavy metals such as mercury if present. Gold can also be associated with arsenopyrite (FeAsS), which is similar to iron pyrite (fool's gold), wherein half of the sulfur atoms are replaced by arsenic. Gold-containing arsenopyrite ores are similarly reactive toward inorganic cyanide.
Industrial organic chemistry
The second major application of alkali metal cyanides (after mining) is in the production of CN-containing compounds, usually nitriles. Acyl cyanides are produced from acyl chlorides and cyanide. Cyanogen, cyanogen chloride, and the trimer cyanuric chloride are derived from alkali metal cyanides.
Medical uses
The cyanide compound sodium nitroprusside is used mainly in clinical chemistry to measure urine ketone bodies mainly as a follow-up to diabetic patients. On occasion, it is used in emergency medical situations to produce a rapid decrease in blood pressure in humans; it is also used as a vasodilator in vascular research. The cobalt in artificial vitamin B12 contains a cyanide ligand as an artifact of the purification process; this must be removed by the body before the vitamin molecule can be activated for biochemical use. During World War I, a copper cyanide compound was briefly used by Japanese physicians for the treatment of tuberculosis and leprosy.
Illegal fishing and poaching
Main article: Cyanide fishingCyanides are illegally used to capture live fish near coral reefs for the aquarium and seafood markets. The practice is controversial, dangerous, and damaging but is driven by the lucrative exotic fish market.
Poachers in Africa have been known to use cyanide to poison waterholes, to kill elephants for their ivory.
Pest control
M44 cyanide devices are used in the United States to kill coyotes and other canids. Cyanide is also used for pest control in New Zealand, particularly for possums, an introduced marsupial that threatens the conservation of native species and spreads tuberculosis amongst cattle. Possums can become bait shy but the use of pellets containing the cyanide reduces bait shyness. Cyanide has been known to kill native birds, including the endangered kiwi. Cyanide is also effective for controlling the dama wallaby, another introduced marsupial pest in New Zealand. A licence is required to store, handle and use cyanide in New Zealand.
Cyanides are used as insecticides for fumigating ships. Cyanide salts are used for killing ants, and have in some places been used as rat poison (the less toxic poison arsenic is more common).
Niche uses
Potassium ferrocyanide is used to achieve a blue color on cast bronze sculptures during the final finishing stage of the sculpture. On its own, it will produce a very dark shade of blue and is often mixed with other chemicals to achieve the desired tint and hue. It is applied using a torch and paint brush while wearing the standard safety equipment used for any patina application: rubber gloves, safety glasses, and a respirator. The actual amount of cyanide in the mixture varies according to the recipes used by each foundry.
Cyanide is also used in jewelry-making and certain kinds of photography such as sepia toning.
Although usually thought to be toxic, cyanide and cyanohydrins increase germination in various plant species.
Human poisoning
Main article: Cyanide poisoningDeliberate cyanide poisoning of humans has occurred many times throughout history. Common salts such as sodium cyanide are involatile but water-soluble, so are poisonous by ingestion. Hydrogen cyanide is a gas, making it more indiscriminately dangerous, however it is lighter than air and rapidly disperses up into the atmosphere, which makes it ineffective as a chemical weapon.
Food additive
Because of the high stability of their complexation with iron, ferrocyanides (Sodium ferrocyanide E535, Potassium ferrocyanide E536, and Calcium ferrocyanide E538) do not decompose to lethal levels in the human body and are used in the food industry as, e.g., an anticaking agent in table salt.
Chemical tests for cyanide
Cyanide is quantified by potentiometric titration, a method widely used in gold mining. It can also be determined by titration with silver ion. Some analyses begin with an air-purge of an acidified boiling solution, sweeping the vapors into a basic absorber solution. The cyanide salt absorbed in the basic solution is then analyzed.
Qualitative tests
Because of the notorious toxicity of cyanide, many methods have been investigated. Benzidine gives a blue coloration in the presence of ferricyanide. Iron(II) sulfate added to a solution of cyanide, such as the filtrate from the sodium fusion test, gives prussian blue. A solution of para-benzoquinone in DMSO reacts with inorganic cyanide to form a cyanophenol, which is fluorescent. Illumination with a UV light gives a green/blue glow if the test is positive.
References
- "cyanides". IUPAC Gold Book. 2014. doi:10.1351/goldbook.C01486.
- "Environmental and Health Effects of Cyanide". International Cyanide Management Institute. 2006. Archived from the original on 30 November 2012. Retrieved 4 August 2009.
- Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
- G. L. Miessler and D. A. Tarr "Inorganic Chemistry" 3rd Ed, Pearson/Prentice Hall publisher, ISBN 0-13-035471-6.
- "ToxFAQs for Cyanide". Agency for Toxic Substances and Disease Registry. July 2006. Retrieved 2008-06-28.
- Vetter, J. (2000). "Plant cyanogenic glycosides". Toxicon. 38 (1): 11–36. doi:10.1016/S0041-0101(99)00128-2. PMID 10669009.
- Jones, D. A. (1998). "Why are so many food plants cyanogenic?". Phytochemistry. 47 (2): 155–162. Bibcode:1998PChem..47..155J. doi:10.1016/S0031-9422(97)00425-1. PMID 9431670.
- Reissmann, Stefanie; Hochleitner, Elisabeth; Wang, Haofan; Paschos, Athanasios; Lottspeich, Friedrich; Glass, Richard S.; Böck, August (2003). "Taming of a Poison: Biosynthesis of the NiFe-Hydrogenase Cyanide Ligands" (PDF). Science. 299 (5609): 1067–1070. Bibcode:2003Sci...299.1067R. doi:10.1126/science.1080972. PMID 12586941. S2CID 20488694. Archived (PDF) from the original on 2020-11-23.
- Pieniazek, Piotr A.; Bradforth, Stephen E.; Krylov, Anna I. (2005-12-07). "Spectroscopy of the Cyano Radical in an Aqueous Environment" (PDF). The Journal of Physical Chemistry A. 110 (14): 4854–4865. Bibcode:2006JPCA..110.4854P. doi:10.1021/jp0545952. PMID 16599455. Archived from the original (PDF) on 2008-09-11. Retrieved 2008-08-23.
- Roth, K. C.; Meyer, D. M.; Hawkins, I. (1993). "Interstellar Cyanogen and the Temperature of the Cosmic Microwave Background Radiation" (PDF). The Astrophysical Journal. 413 (2): L67–L71. Bibcode:1993ApJ...413L..67R. doi:10.1086/186961.
- ^ Anon (June 27, 2013). "Facts about cyanide:Where cyanide is found and how it is used". CDC Emergency preparedness and response. Centers for Disease Control and Prevention. Retrieved 10 December 2016.
- IUPAC Gold Book nitriles
- NCBI-MeSH Nitriles
- ^ Rubo, Andreas; Kellens, Raf; Reddy, Jay; Steier, Norbert; Hasenpusch, Wolfgang (2006). "Alkali Metal Cyanides". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.i01_i01. ISBN 978-3527306732.
- Pollak, Peter; Romeder, Gérard; Hagedorn, Ferdinand; Gelbke, Heinz-Peter (2000). "Nitriles". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_363. ISBN 3-527-30673-0.
- Young, C. A., & Jordan, T. S. (1995, May). Cyanide remediation: current and past technologies. In: Proceedings of the 10th Annual Conference on Hazardous Waste Research (pp. 104–129). Kansas State University: Manhattan, KS. https://engg.ksu.edu/HSRC/95Proceed/young.pdf
- Dmitry Yermakov. "Cyanide Destruction | SRK Consulting". srk.com. Retrieved 2 March 2021.
- Botz Michael M. Overview of cyanide treatment methods. Elbow Creek Engineering, Inc. http://www.botz.com/MEMCyanideTreatment.pdf
- Sharpe, A. G. The Chemistry of Cyano Complexes of the Transition Metals; Academic Press: London, 1976
- Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 978-0-12-352651-9.
- Andrussow, Leonid (1927). "Über die schnell verlaufenden katalytischen Prozesse in strömenden Gasen und die Ammoniak-Oxydation (V)" [About the quicka catalytic processes in flowing gases and the ammonia oxidation (V)]. Berichte der Deutschen Chemischen Gesellschaft (in German). 60 (8): 2005–2018. doi:10.1002/cber.19270600857.
- Andrussow, L. (1935). "Über die katalytische Oxydation von Ammoniak-Methan-Gemischen zu Blausäure" [About the catalytic oxidation of ammonia-methane mixtures to cyanide]. Angewandte Chemie (in German). 48 (37): 593–595. Bibcode:1935AngCh..48..593A. doi:10.1002/ange.19350483702.
- Nelson, David L.; Cox, Michael M. (2000). Lehniger Principles of Biochemistry (3rd ed.). New York: Worth Publishers. pp. 668, 670–71, 676. ISBN 978-1-57259-153-0.
- ^ Biller, José (2007). "163". Interface of neurology and internal medicine (illustrated ed.). Lippincott Williams & Wilkins. p. 939. ISBN 978-0-7817-7906-7.
- "MSDS of trimethylsilyl cyanide" (PDF). Gelest Inc. 2008. Archived (PDF) from the original on 2022-10-10. Retrieved 2022-08-16.
- Cyanide Toxicity~treatment at eMedicine
- Chaudhary, M.; Gupta, R. (2012). "Cyanide Detoxifying Enzyme: Rhodanese". Current Biotechnology. 1 (4): 327–335. doi:10.2174/2211550111201040327.
- Toxicological Profile for Cyanide (PDF) (Report). U.S. Department of Health and Human Services. 2006. pp. 18–19. Archived (PDF) from the original on 2004-03-31.
- Kumar, Rahul; Saha, Shouvik; Sarita, Dhaka; Mayur B., Kurade; Kang, Chan Ung; Baek, Seung Han; Jeong, Byong-Hun (2016). "Remediation of cyanide-contaminated environments through microbes and plants: a review of current knowledge and future perspectives". Geosystem Engineering. 70 (1): 28–40. doi:10.1080/12269328.2016.1218303. S2CID 132571397. Retrieved 24 April 2022.
- Konyratbekova, Saltanat Sabitovna; Baikonurova, Aliya; Akcil, Ata (2015-05-04). "Non-cyanide Leaching Processes in Gold Hydrometallurgy and Iodine-Iodide Applications: A Review". Mineral Processing and Extractive Metallurgy Review. 36 (3): 198–212. doi:10.1080/08827508.2014.942813. ISSN 0882-7508.
- Zhang, Yan; Cui, Mingyao; Wang, Jianguo; Liu, Xiaoliang; Lyu, Xianjun (2022). "A review of gold extraction using alternatives to cyanide: Focus on current status and future prospects of the novel eco-friendly synthetic gold lixiviants". Minerals Engineering. 176: 107336. doi:10.1016/j.mineng.2021.107336.
- Takano, R. (August 1916). "The treatment of leprosy with cyanocuprol". The Journal of Experimental Medicine. 24 (2): 207–211. doi:10.1084/jem.24.2.207. PMC 2125457. PMID 19868035. Retrieved 2008-06-28.
- Dzombak, David A; Ghosh, Rajat S; Wong-Chong, George M. Cyanide in Water and Soil. CRC Press, 2006, Chapter 11.2: "Use of Cyanide for Capturing Live Reef Fish".
- Poachers kill 80 elephants with cyanide in Zimbabwe ABC News, 25 September 2013. Retrieved 30 October 2015.
- Shivik, John A.; Mastro, Lauren; Young, Julie K. (2014). "Animal attendance at M-44 sodium cyanide ejector sites for coyotes". Wildlife Society Bulletin. 38 (1): 217–220. Bibcode:2014WSBu...38..217S. doi:10.1002/wsb.361.
- Green, Wren (July 2004). "The use of 1080 for pest control" (PDF). New Zealand Department of Conservation. Retrieved 8 June 2011.
- Shapiro, Lee; et al. (21 March 2011). "Effectiveness of cyanide pellets for control of dama wallabies (Macropus eugenii)" (PDF). New Zealand Journal of Ecology. 35 (3). Archived (PDF) from the original on 2015-02-03.
- "Sodium Cyanide". PubChem. National Center for Biotechnology Information. 2016. Retrieved 2 September 2016.
Cyanide and hydrogen cyanide are used in electroplating, metallurgy, organic chemicals production, photographic developing, manufacture of plastics, fumigation of ships, and some mining processes.
- "Reregistration Eligibility Decision (RED) Sodium Cyanide" (PDF). EPA.gov. 1 September 1994. p. 7. Archived (PDF) from the original on 2022-10-10. Retrieved 2 September 2016.
Sodium cyanide was initially registered as a pesticide on December 23, 1947, to control ants on uncultivated agricultural and non-agricultural areas.
- "Tariff Information, 1921: Hearings on General Tariff Revision Before the Committee on Ways and Means, House of Representatives". AbeBooks.com. US Congress, House Committee on Ways and Means, US Government Printing Office. 1921. p. 3987. Retrieved 2 September 2016.
Another field in which cyanide is used in growing quantity is the eradication of rats and other vermin – especially in the fight against typhus.
- "Deadliest Poisons Used by Man". PlanetDeadly.com. 18 November 2013. Archived from the original on 11 May 2016. Retrieved 2 September 2016.
- Taylorson, R.; Hendricks, SB (1973). "Promotion of Seed Germination by Cyanide". Plant Physiol. 52 (1): 23–27. doi:10.1104/pp.52.1.23. PMC 366431. PMID 16658492.
- Mullick, P.; Chatterji, U. N. (1967). "Effect of sodium cyanide on germination of two leguminous seeds". Plant Systematics and Evolution. 114: 88–91. doi:10.1007/BF01373937. S2CID 2533762.
- Bernan (2008). Medical Management of Chemical Casualties Handbook (4th ed.). Government Printing Off. p. 41. ISBN 978-0-16-081320-7., Extract p. 41
- Bender, David A.; Bender, Arnold Eric (1997). Benders' dictionary of nutrition and food technology (7th ed.). Woodhead Publishing. p. 459. ISBN 978-1-85573-475-3. Extract of page 459
- Schulz, Horst D.; Hadeler, Astrid; Deutsche Forschungsgemeinschaft (2003). Geochemical processes in soil and groundwater: measurement – modelling – upscaling. Wiley-VCH. p. 67. doi:10.1002/9783527609703. ISBN 978-3-527-27766-7.
- Gail, Ernst; Gos, Stephen; Kulzer, Rupprecht; Lorösch, Jürgen; Rubo, Andreas; Sauer, Manfred (2004). "Cyano Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_159.pub2. ISBN 978-3527306732.
- Schwenecke, H.; Mayer, D. (2005). "Benzidine and Benzidine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_539. ISBN 978-3527306732.
- Ganjeloo, A; Isom, GE; Morgan, RL; Way, JL (1980). "Fluorometric determination of cyanide in biological fluids with p-benzoquinone*1". Toxicology and Applied Pharmacology. 55 (1): 103–107. doi:10.1016/0041-008X(80)90225-2. PMID 7423496.
External links
- ATSDR medical management guidelines for cyanide poisoning (US)
- HSE recommendations for first aid treatment of cyanide poisoning (UK)
- Hydrogen cyanide and cyanides (CICAD 61)
- IPCS/CEC Evaluation of antidotes for poisoning by cyanides
- National Pollutant Inventory – Cyanide compounds fact sheet
- Eating apple seeds is safe despite the small amount of cyanide
- Toxicological Profile for Cyanide, U.S. Department of Health and Human Services, July 2006
- Safety data (French)
- Institut national de recherche et de sécurité (1997). "Cyanure d'hydrogène et solutions aqueuses". Fiche toxicologique n° 4, Paris: INRS, 5 pp. (PDF file, (in French))
- Institut national de recherche et de sécurité (1997). "Cyanure de sodium. Cyanure de potassium". Fiche toxicologique n° 111, Paris: INRS, 6 pp. (PDF file, (in French))
| Salts and covalent derivatives of the cyanide ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inorganic compounds of carbon and related ions | |
|---|---|
| Compounds | |
| Carbon ions | |
| Nanostructures | |
| Oxides and related | |
| Nitrogen species | |
|---|---|
| Hydrides | |
| Organic | |
| Oxides | |
| Halides | |
| Oxidation states | −3, −2, −1, 0, +1, +2, +3, +4, +5 (a strongly acidic oxide) |
| Pest control: Rodenticides | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anticoagulants / Vitamin K antagonists |
| ||||||||
| Convulsants | |||||||||
| Calciferols | |||||||||
| Inorganic compounds | |||||||||
| Organochlorine | |||||||||
| Organophosphorus | |||||||||
| Carbamates | |||||||||
| Others | |||||||||

