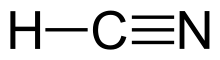
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name Formonitrile | |||
| Systematic IUPAC name Methanenitrile | |||
Other names
| |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| 3DMet | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.747 | ||
| EC Number |
| ||
| KEGG | |||
| MeSH | Hydrogen+Cyanide | ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1051 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | HCN | ||
| Molar mass | 27.0253 g/mol | ||
| Appearance | Colorless liquid or gas | ||
| Odor | bitter almond-like | ||
| Density | 0.6876 g/cm | ||
| Melting point | −13.29 °C (8.08 °F; 259.86 K) | ||
| Boiling point | 26 °C (79 °F; 299 K) | ||
| Solubility in water | Miscible | ||
| Solubility in ethanol | Miscible | ||
| Vapor pressure | 100 kPa (25 °C) | ||
| Henry's law constant (kH) |
75 μmol Pa kg | ||
| Acidity (pKa) | 9.21 (in water),
12.9 (in DMSO) | ||
| Basicity (pKb) | 4.79 (cyanide anion) | ||
| Conjugate acid | Hydrocyanonium | ||
| Conjugate base | Cyanide | ||
| Refractive index (nD) | 1.2675 | ||
| Viscosity | 0.183 mPa·s (25 °C) | ||
| Structure | |||
| Crystal structure | tetragonal (>170 K) orthorhombic (<170 K) | ||
| Point group | C∞v | ||
| Molecular shape | Linear | ||
| Dipole moment | 2.98 D | ||
| Thermochemistry | |||
| Heat capacity (C) | 35.9 J K mol (gas) | ||
| Std molar entropy (S298) |
201.8 J K mol | ||
| Std enthalpy of formation (ΔfH298) |
135.1 kJ mol | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |    
| ||
| Signal word | Danger | ||
| Hazard statements | H225, H300+H310+H330, H319, H336, H370, H410 | ||
| Precautionary statements | P210, P261, P305+P351+P338 | ||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | −17.8 °C (0.0 °F; 255.3 K) | ||
| Autoignition temperature |
538 °C (1,000 °F; 811 K) | ||
| Explosive limits | 5.6% – 40.0% | ||
| Lethal dose or concentration (LD, LC): | |||
| LC50 (median concentration) | 501 ppm (rat, 5 min) 323 ppm (mouse, 5 min) 275 ppm (rat, 15 min) 170 ppm (rat, 30 min) 160 ppm (rat, 30 min) 323 ppm (rat, 5 min) | ||
| LCLo (lowest published) | 200 ppm (mammal, 5 min) 36 ppm (mammal, 2 hr) 107 ppm (human, 10 min) 759 ppm (rabbit, 1 min) 759 ppm (cat, 1 min) 357 ppm (human, 2 min) 179 ppm (human, 1 hr) | ||
| NIOSH (US health exposure limits): | |||
| PEL (Permissible) | TWA 10 ppm (11 mg/m) | ||
| REL (Recommended) | ST 4.7 ppm (5 mg/m) | ||
| IDLH (Immediate danger) | 50 ppm | ||
| Related compounds | |||
| Related alkanenitriles | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Hydrogen cyanide (formerly known as prussic acid) is a chemical compound with the formula HCN and structural formula H−C≡N. It is a highly toxic and flammable liquid that boils slightly above room temperature, at 25.6 °C (78.1 °F). HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its volatile nature. A solution of hydrogen cyanide in water, represented as HCN, is called hydrocyanic acid. The salts of the cyanide anion are known as cyanides.
Whether hydrogen cyanide is an organic compound or not is a topic of debate among chemists, and opinions vary from author to author. Traditionally, it is considered inorganic by a significant number of authors. Contrary to this view, it is considered organic by other authors, because hydrogen cyanide belongs to the class of organic compounds known as nitriles which have the formula R−C≡N, where R is typically organyl group (e.g., alkyl or aryl) or hydrogen. In the case of hydrogen cyanide, the R group is hydrogen H, so the other names of hydrogen cyanide are methanenitrile and formonitrile.
Structure and general properties
Hydrogen cyanide is a linear molecule, with a triple bond between carbon and nitrogen. The tautomer of HCN is HNC, hydrogen isocyanide.
HCN has a faint bitter almond-like odor that some people are unable to detect owing to a recessive genetic trait. The volatile compound has been used as inhalation rodenticide and human poison, as well as for killing whales. Cyanide ions interfere with iron-containing respiratory enzymes.
Chemical properties
Hydrogen cyanide is weakly acidic with a pKa of 9.2. It partially ionizes in water to give the cyanide anion, CN. HCN forms hydrogen bonds with its conjugate base, species such as (CN)(HCN)n.
Hydrogen cyanide reacts with alkenes to give nitriles. The conversion, which is called hydrocyanation, employs nickel complexes as catalysts.
- RCH=CH2 + HCN → RCH2−CH2CN
Four molecules of HCN will tetramerize into diaminomaleonitrile.
Metal cyanides are typically prepared by salt metathesis from alkali metal cyanide salts, but mercuric cyanide is formed from aqueous hydrogen cyanide:
- HgO + 2 HCN → Hg(CN)2 + H2O
History of discovery and naming
Hydrogen cyanide was first isolated in 1752 by French chemist Pierre Macquer who converted Prussian blue to an iron oxide plus a volatile component and found that these could be used to reconstitute it. The new component was what is now known as hydrogen cyanide. It was subsequently prepared from Prussian blue by the Swedish chemist Carl Wilhelm Scheele in 1782, and was eventually given the German name Blausäure (lit. "Blue acid") because of its acidic nature in water and its derivation from Prussian blue. In English, it became known popularly as prussic acid.
In 1787, the French chemist Claude Louis Berthollet showed that prussic acid did not contain oxygen, an important contribution to acid theory, which had hitherto postulated that acids must contain oxygen (hence the name of oxygen itself, which is derived from Greek elements that mean "acid-former" and are likewise calqued into German as Sauerstoff).
In 1811, Joseph Louis Gay-Lussac prepared pure, liquified hydrogen cyanide, and in 1815 he deduced Prussic acid's chemical formula.
Etymology
The word cyanide for the radical in hydrogen cyanide was derived from its French equivalent, cyanure, which Gay-Lussac constructed from the Ancient Greek word κύανος for dark blue enamel or lapis lazuli, again owing to the chemical’s derivation from Prussian blue. Incidentally, the Greek word is also the root of the English color name cyan.
Production and synthesis
The most important process is the Andrussow oxidation invented by Leonid Andrussow at IG Farben in which methane and ammonia react in the presence of oxygen at about 1,200 °C (2,190 °F) over a platinum catalyst:
- 2 CH4 + 2 NH3 + 3 O2 → 2 HCN + 6 H2O
In 2006, between 500 million and 1 billion pounds (between 230,000 and 450,000 t) were produced in the US. Hydrogen cyanide is produced in large quantities by several processes and is a recovered waste product from the manufacture of acrylonitrile.
Of lesser importance is the Degussa process (BMA process) in which no oxygen is added and the energy must be transferred indirectly through the reactor wall:
- CH4 + NH3 → HCN + 3H2
This reaction is akin to steam reforming, the reaction of methane and water to give carbon monoxide and hydrogen.
In the Shawinigan Process, hydrocarbons, e.g. propane, are reacted with ammonia.
In the laboratory, small amounts of HCN are produced by the addition of acids to cyanide salts of alkali metals:
- H + CN → HCN
This reaction is sometimes the basis of accidental poisonings because the acid converts a nonvolatile cyanide salt into the gaseous HCN.
Hydrogen cyanide could be obtained from potassium ferricyanide and acid:
- 6 H + [Fe(CN)6]−3 → 6 HCN + Fe+3
Historical methods of production
The large demand for cyanides for mining operations in the 1890s was met by George Thomas Beilby, who patented a method to produce hydrogen cyanide by passing ammonia over glowing coal in 1892. This method was used until Hamilton Castner in 1894 developed a synthesis starting from coal, ammonia, and sodium yielding sodium cyanide, which reacts with acid to form gaseous HCN.
Applications
HCN is the precursor to sodium cyanide and potassium cyanide, which are used mainly in gold and silver mining and for the electroplating of those metals. Via the intermediacy of cyanohydrins, a variety of useful organic compounds are prepared from HCN including the monomer methyl methacrylate, from acetone, the amino acid methionine, via the Strecker synthesis, and the chelating agents EDTA and NTA. Via the hydrocyanation process, HCN is added to butadiene to give adiponitrile, a precursor to Nylon-6,6.
HCN is used globally as a fumigant against many species of pest insects that infest food production facilities. Both its efficacy and method of application lead to very small amounts of the fumigant being used compared to other toxic substances used for the same purpose. Using HCN as a fumigant also has less environmental impact, compared to some other fumigants such as sulfuryl fluoride, and methyl bromide.
Occurrence
HCN is obtainable from fruits that have a pit, such as cherries, apricots, apples, and nuts such as bitter almonds, from which almond oil and extract is made. Many of these pits contain small amounts of cyanohydrins such as mandelonitrile and amygdalin, which slowly release hydrogen cyanide. One hundred grams of crushed apple seeds can yield about 70 mg of HCN. The roots of cassava plants contain cyanogenic glycosides such as linamarin, which decompose into HCN in yields of up to 370 mg per kilogram of fresh root. Some millipedes, such as Harpaphe haydeniana, Desmoxytes purpurosea, and Apheloria release hydrogen cyanide as a defense mechanism, as do certain insects, such as burnet moths and the larvae of Paropsisterna eucalyptus. Hydrogen cyanide is contained in the exhaust of vehicles, and in smoke from burning nitrogen-containing plastics.

On Titan
HCN has been measured in Titan's atmosphere by four instruments on the Cassini space probe, one instrument on Voyager, and one instrument on Earth. One of these measurements was in situ, where the Cassini spacecraft dipped between 1,000 and 1,100 km (620 and 680 mi) above Titan's surface to collect atmospheric gas for mass spectrometry analysis. HCN initially forms in Titan's atmosphere through the reaction of photochemically produced methane and nitrogen radicals which proceed through the H2CN intermediate, e.g., (CH3 + N → H2CN + H → HCN + H2). Ultraviolet radiation breaks HCN up into CN + H; however, CN is efficiently recycled back into HCN via the reaction CN + CH4 → HCN + CH3.
On the young Earth
It has been postulated that carbon from a cascade of asteroids (known as the Late Heavy Bombardment), resulting from interaction of Jupiter and Saturn, blasted the surface of young Earth and reacted with nitrogen in Earth's atmosphere to form HCN.
In mammals
Some authors have shown that neurons can produce hydrogen cyanide upon activation of their opioid receptors by endogenous or exogenous opioids. They have also shown that neuronal production of HCN activates NMDA receptors and plays a role in signal transduction between neuronal cells (neurotransmission). Moreover, increased endogenous neuronal HCN production under opioids was seemingly needed for adequate opioid analgesia, as analgesic action of opioids was attenuated by HCN scavengers. They considered endogenous HCN to be a neuromodulator.
It has also been shown that, while stimulating muscarinic cholinergic receptors in cultured pheochromocytoma cells increases HCN production, in a living organism (in vivo) muscarinic cholinergic stimulation actually decreases HCN production.
Leukocytes generate HCN during phagocytosis, and can kill bacteria, fungi, and other pathogens by generating several different toxic chemicals, one of which is hydrogen cyanide.
The vasodilatation caused by sodium nitroprusside has been shown to be mediated not only by NO generation, but also by endogenous cyanide generation, which adds not only toxicity, but also some additional antihypertensive efficacy compared to nitroglycerine and other non-cyanogenic nitrates which do not cause blood cyanide levels to rise.
HCN is a constituent of tobacco smoke.
HCN and the origin of life
Hydrogen cyanide has been discussed as a precursor to amino acids and nucleic acids, and is proposed to have played a part in the origin of life. Although the relationship of these chemical reactions to the origin of life theory remains speculative, studies in this area have led to discoveries of new pathways to organic compounds derived from the condensation of HCN (e.g. Adenine).
In space
See also: AstrochemistryHCN has been detected in the interstellar medium and in the atmospheres of carbon stars. Since then, extensive studies have probed formation and destruction pathways of HCN in various environments and examined its use as a tracer for a variety of astronomical species and processes. HCN can be observed from ground-based telescopes through a number of atmospheric windows. The J=1→0, J=3→2, J= 4→3, and J=10→9 pure rotational transitions have all been observed.
HCN is formed in interstellar clouds through one of two major pathways: via a neutral-neutral reaction (CH2 + N → HCN + H) and via dissociative recombination (HCNH + e → HCN + H). The dissociative recombination pathway is dominant by 30%; however, the HCNH must be in its linear form. Dissociative recombination with its structural isomer, H2NC, exclusively produces hydrogen isocyanide (HNC).
HCN is destroyed in interstellar clouds through a number of mechanisms depending on the location in the cloud. In photon-dominated regions (PDRs), photodissociation dominates, producing CN (HCN + ν → CN + H). At further depths, photodissociation by cosmic rays dominate, producing CN (HCN + cr → CN + H). In the dark core, two competing mechanisms destroy it, forming HCN and HCNH (HCN + H → HCN + H; HCN + HCO → HCNH + CO). The reaction with HCO dominates by a factor of ~3.5. HCN has been used to analyze a variety of species and processes in the interstellar medium. It has been suggested as a tracer for dense molecular gas and as a tracer of stellar inflow in high-mass star-forming regions. Further, the HNC/HCN ratio has been shown to be an excellent method for distinguishing between PDRs and X-ray-dominated regions (XDRs).
On 11 August 2014, astronomers released studies, using the Atacama Large Millimeter/Submillimeter Array (ALMA) for the first time, that detailed the distribution of HCN, HNC, H2CO, and dust inside the comae of comets C/2012 F6 (Lemmon) and C/2012 S1 (ISON).
In February 2016, it was announced that traces of hydrogen cyanide were found in the atmosphere of the hot Super-Earth 55 Cancri e with NASA's Hubble Space Telescope.
On 14 December 2023, astronomers reported the first time discovery, in the plumes of Enceladus, moon of the planet Saturn, of hydrogen cyanide, a possible chemical essential for life as we know it, as well as other organic molecules, some of which are yet to be better identified and understood. According to the researchers, "these compounds could potentially support extant microbial communities or drive complex organic synthesis leading to the origin of life."
As a poison and chemical weapon
Main article: Cyanide poisoningIn World War I, hydrogen cyanide was used by the French from 1916 as a chemical weapon against the Central Powers, and by the United States and Italy in 1918. It was not found to be effective enough due to weather conditions. The gas is lighter than air and rapidly disperses up into the atmosphere. Rapid dilution made its use in the field impractical. In contrast, denser agents such as phosgene or chlorine tended to remain at ground level and sank into the trenches of the Western Front's battlefields. Compared to such agents, hydrogen cyanide had to be present in higher concentrations in order to be fatal.
A hydrogen cyanide concentration of 100–200 ppm in breathing air will kill a human within 10 to 60 minutes. A hydrogen cyanide concentration of 2000 ppm (about 2380 mg/m) will kill a human in about one minute. The toxic effect is caused by the action of the cyanide ion, which halts cellular respiration. It acts as a non-competitive inhibitor for an enzyme in mitochondria called cytochrome c oxidase. As such, hydrogen cyanide is commonly listed among chemical weapons as a blood agent.
The Chemical Weapons Convention lists it under Schedule 3 as a potential weapon which has large-scale industrial uses. Signatory countries must declare manufacturing plants that produce more than 30 metric tons per year, and allow inspection by the Organisation for the Prohibition of Chemical Weapons.
Perhaps its most infamous use is Zyklon B (German: Cyclone B, with the B standing for Blausäure – prussic acid; also, to distinguish it from an earlier product later known as Zyklon A), used in Nazi German extermination camps during World War II to kill Jews and other persecuted minorities en masse as part of their Final Solution genocide program. Hydrogen cyanide was also used in the camps for delousing clothing in attempts to eradicate diseases carried by lice and other parasites. One of the original Czech producers continued making Zyklon B under the trademark "Uragan D2" until around 2015.
During World War II, the US considered using it, along with cyanogen chloride, as part of Operation Downfall, the planned invasion of Japan, but President Harry Truman decided against it, instead using the atomic bombs developed by the secret Manhattan Project.
Hydrogen cyanide was also the agent employed in judicial execution in some U.S. states, where it was produced during the execution by the action of sulfuric acid on sodium cyanide or potassium cyanide.
Under the name prussic acid, HCN has been used as a killing agent in whaling harpoons, although it proved quite dangerous to the crew deploying it, and it was quickly abandoned. From the middle of the 18th century it was used in a number of poisoning murders and suicides.
Hydrogen cyanide gas in air is explosive at concentrations above 5.6%.
References
- "hydrogen cyanide (CHEBI:18407)". Chemical Entities of Biological Interest. UK: European Bioinformatics Institute. 18 October 2009. Main. Retrieved 2012-06-04.
- ^ "Hydrogen Cyanide". PubChem. National Center for Biotechnology Information.
- Simeonova, Fina Petrova; Fishbein, Lawrence (2004). Hydrogen cyanide and cyanides : human health aspects (Report). World Health Organization. ISBN 9241530618. ISSN 1020-6167.
- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. ISBN 978-1439855119.
- Evans DA. "pKa's of Inorganic and Oxo-Acids" (PDF). Archived (PDF) from the original on 2022-10-09. Retrieved June 19, 2020.
- Patnaik P (2002). Handbook of Inorganic Chemicals. McGraw-Hill. ISBN 978-0070494398.
- Schulz, Axel; Surkau, Jonas (2022-09-21). "Main group cyanides: from hydrogen cyanide to cyanido-complexes". Reviews in Inorganic Chemistry. 43 (1). Walter de Gruyter GmbH: 49–188. doi:10.1515/revic-2021-0044. ISSN 0193-4929.
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0333". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Hydrogen cyanide". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Gail, E.; Gos, S.; Kulzer, R.; Lorösch, J.; Rubo, A.; Sauer, M. "Cyano Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_159.pub2. ISBN 978-3527306732.
- "Human Metabolome Database: Showing metabocard for Hydrogen cyanide (HMDB0060292)".
- "Cyanide, inability to smell". Online Mendelian Inheritance in Man. Retrieved 2010-03-31.
- ^ Lytle T. "Poison Harpoons". Whalecraft.net. Archived from the original on 2019-02-15.
- Bläsing, Kevin; Harloff, Jörg; Schulz, Axel; Stoffers, Alrik; Stoer, Philip; Villinger, Alexander (2020). "Salts of HCN-Cyanide Aggregates: [CN(HCN)2] and [CN(HCN)3]". Angewandte Chemie International Edition. 59 (26): 10508–10513. doi:10.1002/anie.201915206. PMC 7317722. PMID 32027458.
- Leeuwen, P. W. N. M. van (2004). Homogeneous Catalysis: Understanding the Art. Dordrecht: Kluwer Academic Publishers. ISBN 1402019998. OCLC 54966334.
- Ferris, J. P.; Sanchez, R. A. (1968). "Diaminomaleonitrile (Hydrogen Cyanide Tetramer)". Organic Syntheses. 48: 60. doi:10.15227/orgsyn.048.0060.
- F. Wagenknecht; R. Juza (1963). "Mercury (II) cyanide". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry. Vol. 2 (2nd ed.). NY, NY: Academic Press.
- Macquer PJ (1756). "Éxamen chymique de bleu de Prusse" [Chemical examination of Prussian blue]. Mémoires de l'Académie royale des Sciences (in French): 60–77.
- Scheele CW (1782). "Försök, beträffande det färgande ämnet uti Berlinerblå" [Experiment concerning the coloring substance in Berlin blue]. Kungliga Svenska Vetenskapsakademiens Handlingar (Royal Swedish Academy of Science's Proceedings (in Swedish). 3: 264–275.
Reprinted in Latin as: Scheele CW, Hebenstreit EB, eds. (1789). "De materia tingente caerulei berolinensis". Opuscula Chemica et Physica [The dark matter tingente caerulei berolinensis] (in Latin). Vol. 2. Translated by Schäfer GH. (Leipzig ("Lipsiae") (Germany): Johann Godfried Müller. pp. 148–174. - Berthollet CL (1789). "Mémoire sur l'acide prussique" [Memoir on prussic acid]. Mémoires de l'Académie Royale des Sciences (in French): 148–161.
Reprinted in: Berthollet CL (1789). "Extrait d'un mémoire sur l'acide prussique" [Extract of a memoir on prussic acid]. Annales de Chimie. 1: 30–39. - Newbold BT (1999-11-01). "Claude Louis Berthollet: A Great Chemist in the French Tradition". Canadian Chemical News. Archived from the original on 2008-04-20. Retrieved 2010-03-31.
- Gay-Lussac JL (1811). "Note sur l'acide prussique" [Note on prussic acid]. Annales de Chimie. 44: 128–133.
- Gay-Lussac JL (1815). "Recherche sur l'acide prussique" [Research on prussic acid]. Annales de Chimie. 95: 136–231.
- Andrussow L (1935). "The catalytic oxydation of ammonia-methane-mixtures to hydrogen cyanide". Angewandte Chemie. 48 (37): 593–595. Bibcode:1935AngCh..48..593A. doi:10.1002/ange.19350483702.
- "Non-confidential 2006 IUR Records by Chemical, including Manufacturing, Processing and Use Information". EPA. Archived from the original on 2013-05-10. Retrieved 2013-01-31.
- Endter F (1958). "Die technische Synthese von Cyanwasserstoff aus Methan und Ammoniak ohne Zusatz von Sauerstoff". Chemie Ingenieur Technik. 30 (5): 305–310. doi:10.1002/cite.330300506.
- "MSDS for potassium ferricyanide" (PDF). Archived from the original (PDF) on 2016-04-18. Retrieved 2023-04-17.
- "Potassium ferricyanide". PubChem. National Center for Biotechnology Information.
- "Manual of fumigation for insect control – Space fumigation at atmospheric pressure (Cont.)". Food and Agriculture Organization.
- "New greenhouse gas identified". News.mit.edu. 11 March 2009.
- "Chapter 10 : Methyl Bromide" (PDF). Csl.noaa.gov. Archived (PDF) from the original on 2022-10-09.
- Vetter J (January 2000). "Plant cyanogenic glycosides". Toxicon. 38 (1): 11–36. Bibcode:2000Txcn...38...11V. doi:10.1016/S0041-0101(99)00128-2. PMID 10669009.
- Jones DA (January 1998). "Why are so many food plants cyanogenic?". Phytochemistry. 47 (2): 155–162. Bibcode:1998PChem..47..155J. doi:10.1016/S0031-9422(97)00425-1. PMID 9431670.
- "Are Apple Cores Poisonous?". The Naked Scientists. 26 September 2010. Archived from the original on 6 March 2014. Retrieved 6 March 2014.
- Aregheore EM, Agunbiade OO (June 1991). "The toxic effects of cassava (manihot esculenta grantz) diets on humans: a review". Veterinary and Human Toxicology. 33 (3): 274–275. PMID 1650055.
- Blum MS, Woodring JP (October 1962). "Secretion of Benzaldehyde and Hydrogen Cyanide by the Millipede Pachydesmus crassicutis (Wood)". Science. 138 (3539): 512–513. Bibcode:1962Sci...138..512B. doi:10.1126/science.138.3539.512. PMID 17753947. S2CID 40193390.
- Zagrobelny M, de Castro ÉC, Møller BL, Bak S (May 2018). "Cyanogenesis in Arthropods: From Chemical Warfare to Nuptial Gifts". Insects. 9 (2): 51. doi:10.3390/insects9020051. PMC 6023451. PMID 29751568.
- Loison JC, Hébrard E, Dobrijevic M, Hickson KM, Caralp F, Hue V, et al. (February 2015). "The neutral photochemistry of nitriles, amines and imines in the atmosphere of Titan". Icarus. 247: 218–247. Bibcode:2015Icar..247..218L. doi:10.1016/j.icarus.2014.09.039.
- Magee BA, Waite JH, Mandt KE, Westlake J, Bell J, Gell DA (December 2009). "INMS-derived composition of Titan's upper atmosphere: Analysis methods and model comparison". Planetary and Space Science. 57 (14–15): 1895–1916. Bibcode:2009P&SS...57.1895M. doi:10.1016/j.pss.2009.06.016.
- ^ Pearce BK, Molaverdikhani K, Pudritz RE, Henning T, Hébrard E (2020). "HCN Production in Titan's Atmosphere: Coupling Quantum Chemistry and Disequilibrium Atmospheric Modeling". Astrophysical Journal. 901 (2): 110. arXiv:2008.04312. Bibcode:2020ApJ...901..110P. doi:10.3847/1538-4357/abae5c. S2CID 221095540.
- Pearce BK, Ayers PW, Pudritz RE (March 2019). "A Consistent Reduced Network for HCN Chemistry in Early Earth and Titan Atmospheres: Quantum Calculations of Reaction Rate Coefficients". The Journal of Physical Chemistry A. 123 (9): 1861–1873. arXiv:1902.05574. Bibcode:2019JPCA..123.1861P. doi:10.1021/acs.jpca.8b11323. PMID 30721064. S2CID 73442008.
- Wade N (2015-05-04). "Making Sense of the Chemistry That Led to Life on Earth". The New York Times. Retrieved 5 May 2015.
- ^ Borowitz JL, Gunasekar PG, Isom GE (September 1997). "Hydrogen cyanide generation by mu-opiate receptor activation: possible neuromodulatory role of endogenous cyanide". Brain Research. 768 (1–2): 294–300. doi:10.1016/S0006-8993(97)00659-8. PMID 9369328. S2CID 12277593.
- Gunasekar PG, Prabhakaran K, Li L, Zhang L, Isom GE, Borowitz JL (May 2004). "Receptor mechanisms mediating cyanide generation in PC12 cells and rat brain". Neuroscience Research. 49 (1): 13–18. doi:10.1016/j.neures.2004.01.006. PMID 15099699. S2CID 29850349.
- Smith RP, Kruszyna H (January 1976). "Toxicology of some inorganic antihypertensive anions". Federation Proceedings. 35 (1): 69–72. PMID 1245233.
- Talhout R, Schulz T, Florek E, van Benthem J, Wester P, Opperhuizen A (February 2011). "Hazardous compounds in tobacco smoke". International Journal of Environmental Research and Public Health. 8 (2): 613–628. doi:10.3390/ijerph8020613. PMC 3084482. PMID 21556207.
- Ruiz-Bermejo, Marta; Zorzano, María-Paz; Osuna-Esteban, Susana (2013). "Simple Organics and Biomonomers Identified in HCN Polymers: An Overview". Life. 3 (3): 421–448. Bibcode:2013Life....3..421R. doi:10.3390/life3030421. PMC 4187177. PMID 25369814.
- Al-Azmi A, Elassar AZ, Booth BL (2003). "The Chemistry of Diaminomaleonitrile and its Utility in Heterocyclic Synthesis". Tetrahedron. 59 (16): 2749–2763. doi:10.1016/S0040-4020(03)00153-4.
- ^ Snyder LE, Buhl D (1971). "Observations of Radio Emission from Interstellar Hydrogen Cyanide". Astrophysical Journal. 163: L47–L52. Bibcode:1971ApJ...163L..47S. doi:10.1086/180664.
- Jørgensen UG (1997). "Cool Star Models". In van Dishoeck EF (ed.). Molecules in Astrophysics: Probes and Processes. International Astronomical Union Symposia. Molecules in Astrophysics: Probes and Processes. Vol. 178. Springer Science & Business Media. p. 446. ISBN 978-0792345381.
- Treffers RR, Larson HP, Fink U, Gautier TN (1978). "Upper limits to trace constituents in Jupiter's atmosphere from an analysis of its 5-μm spectrum". Icarus. 34 (2): 331–343. Bibcode:1978Icar...34..331T. doi:10.1016/0019-1035(78)90171-9.
- Bieging JH, Shaked S, Gensheimer PD (2000). "Submillimeter- and Millimeter-Wavelength Observations of SiO and HCN in Circumstellar Envelopes of AGB Stars". Astrophysical Journal. 543 (2): 897–921. Bibcode:2000ApJ...543..897B. doi:10.1086/317129.
- Schilke P, Menten KM (2003). "Detection of a Second, Strong Sub-millimeter HCN Laser Line toward Carbon Stars". Astrophysical Journal. 583 (1): 446–450. Bibcode:2003ApJ...583..446S. doi:10.1086/345099. S2CID 122549795.
- ^ Boger GI, Sternberg A (2005). "CN and HCN in Dense Interstellar Clouds". Astrophysical Journal. 632 (1): 302–315. arXiv:astro-ph/0506535. Bibcode:2005ApJ...632..302B. doi:10.1086/432864. S2CID 118958200.
- Gao Y, Solomon PM (2004). "The Star Formation Rate and Dense Molecular Gas in Galaxies". Astrophysical Journal. 606 (1): 271–290. arXiv:astro-ph/0310339. Bibcode:2004ApJ...606..271G. doi:10.1086/382999. S2CID 11335358.
- Gao Y, olomon PM (2004). "HCN Survey of Normal Spiral, Infrared-luminous, and Ultraluminous Galaxies". Astrophysical Journal Supplement Series. 152 (1): 63–80. arXiv:astro-ph/0310341. Bibcode:2004ApJS..152...63G. doi:10.1086/383003. S2CID 9135663.
- Wu J, Evans NJ (2003). "Indications of Inflow Motions in Regions Forming Massive Stars". Astrophysical Journal. 592 (2): L79–L82. arXiv:astro-ph/0306543. Bibcode:2003ApJ...592L..79W. doi:10.1086/377679. S2CID 8016228.
- Loenen AF (2007). "Molecular properties of (U)LIRGs: CO, HCN, HNC and HCO". Proceedings IAU Symposium. 242: 462–466. arXiv:0709.3423. Bibcode:2007IAUS..242..462L. doi:10.1017/S1743921307013609. S2CID 14398456.
- Zubritsky E, Neal-Jones N (11 August 2014). "Release 14-038 – NASA's 3-D Study of Comets Reveals Chemical Factory at Work". NASA. Retrieved 12 August 2014.
- Cordiner MA, Remijan AJ, Boissier J, Milam SN, Mumma MJ, Charnley SB, et al. (11 August 2014). "Mapping the Release of Volatiles in the Inner Comae of Comets C/2012 F6 (Lemmon) and C/2012 S1 (ISON) Using the Atacama Large Millimeter/Submillimeter Array". The Astrophysical Journal. 792 (1): L2. arXiv:1408.2458. Bibcode:2014ApJ...792L...2C. doi:10.1088/2041-8205/792/1/L2. S2CID 26277035.
- "First detection of super-earth atmosphere". ESA/Hubble Information Centre. February 16, 2016.
- Green, Jaime (5 December 2023). "What Is Life? - The answer matters in space exploration. But we still don't really know". The Atlantic. Archived from the original on 5 December 2023. Retrieved 15 December 2023.
- Chang, Kenneth (14 December 2023). "Poison Gas Hints at Potential for Life on an Ocean Moon of Saturn - A researcher who has studied the icy world said "the prospects for the development of life are getting better and better on Enceladus."". The New York Times. Archived from the original on 14 December 2023. Retrieved 15 December 2023.
- Peter, Jonah S.; et al. (14 December 2023). "Detection of HCN and diverse redox chemistry in the plume of Enceladus". Nature Astronomy. 8 (2): 164–173. arXiv:2301.05259. Bibcode:2024NatAs...8..164P. doi:10.1038/s41550-023-02160-0. S2CID 255825649. Archived from the original on 15 December 2023. Retrieved 15 December 2023.
- Schnedlitz, Markus (2008) Chemische Kampfstoffe: Geschichte, Eigenschaften, Wirkung. GRIN Verlag. p. 13. ISBN 3640233603.
- Weapons of War - Poison Gas. firstworldwar.com
- ^ Environmental and Health Effects Archived 2012-11-30 at the Wayback Machine. Cyanidecode.org. Retrieved on 2012-06-02.
- "Hydrogen Cyanide". Organisation for the Prohibition of Chemical Weapons. Retrieved 2009-01-14.
- Van Pelt, Robert Jan; Dwork, Debórah (1996). Auschwitz, 1270 to the present. Norton. p. 443. ISBN 9780300067552.
- "Blue Fume". Chemical Factory Draslovka a.s. Retrieved 2020-07-06.
- "Uragan D2". 2015-07-17. Archived from the original on 2015-07-17. Retrieved 2022-10-19.
- Binkov's Battlegrounds (April 27, 2022). "How would have WW2 gone if the US had not used nuclear bombs on Japan?". YouTube.Com. Retrieved June 23, 2022.
- Pilkington, Ed (28 May 2021). "Arizona 'refurbishes' its gas chamber to prepare for executions, documents reveal". The Guardian. Retrieved 2022-06-14.
- "The Poison Garden website". Thepoisongarden.co.uk. Archived from the original on 10 February 2020. Retrieved 18 October 2014.
- "Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHs) – 74908". NIOSH. 2 November 2018.
External links
- Institut national de recherche et de sécurité (1997). "Cyanure d'hydrogène et solutions aqueuses". Fiche toxicologique n° 4, Paris:INRS, 5pp. (PDF file, in French)
- International Chemical Safety Card 0492
- Hydrogen cyanide and cyanides (CICAD 61)
- National Pollutant Inventory: Cyanide compounds fact sheet
- NIOSH Pocket Guide to Chemical Hazards
- Department of health review
- Density of Hydrogen Cyanide gas
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood agents |
| ||||||||||||||
| Blister agents |
| ||||||||||||||
| Nerve agents |
| ||||||||||||||
| Neurotoxins |
| ||||||||||||||
| Pulmonary/ choking agents |
| ||||||||||||||
| Vomiting agents | |||||||||||||||
| Incapacitating agents |
| ||||||||||||||
| Lachrymatory agents |
| ||||||||||||||
| Malodorant agents | |||||||||||||||
| Cornea-clouding agents | |||||||||||||||
| Biological toxins |
| ||||||||||||||
| Other |
| ||||||||||||||
| Salts and covalent derivatives of the cyanide ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nitrogen species | |
|---|---|
| Hydrides | |
| Organic | |
| Oxides | |
| Halides | |
| Oxidation states | −3, −2, −1, 0, +1, +2, +3, +4, +5 (a strongly acidic oxide) |
| Hydrogen compounds | |
|---|---|
|


