| Revision as of 19:14, 19 December 2023 edit2a02:2f0f:b110:b500:255d:77be:c2e6:bb2a (talk) →Legal status: removed tags for sections that are now tagged← Previous edit | Latest revision as of 00:56, 25 December 2024 edit undoDMacks (talk | contribs)Edit filter managers, Autopatrolled, Administrators186,226 edits No, you changed facts and broke the whole page layout. Undid revision 1265080459 by NW49ERSFAN24 (talk)Tag: Undo | ||
| (55 intermediate revisions by 36 users not shown) | |||
| Line 2: | Line 2: | ||
| {{Other uses}} | {{Other uses}} | ||
| {{Use dmy dates|date=June 2023}} | {{Use dmy dates|date=June 2023}} | ||
| {{cs1 config|name-list-style=vanc|display-authors=6}} | |||
| {{Drugbox | {{Drugbox | ||
| | Verifiedfields |
| Verifiedfields = changed | ||
| | Watchedfields |
| Watchedfields = changed | ||
| | verifiedrevid |
| verifiedrevid = 456481929 | ||
| ⚫ | | image = Codein - Codeine.svg | ||
| | drug_name = | |||
| | |
| width = | ||
| ⚫ | | alt = Skeletal formula | ||
| | type = <!-- empty --> | |||
| | image2 = Codeine molecule ball.png | |||
| ⚫ | | image |
||
| | |
| width2 = | ||
| ⚫ | | alt2 = Ball-and-stick model | ||
| ⚫ | | alt |
||
| ⚫ | | caption = <!-- Clinical data --> | ||
| | image2 = Codeine-from-xtal-Mercury-3D-bs.png | |||
| ⚫ | | pronounce = {{IPAc-en|ˈ|k|oʊ|d|iː|n}} | ||
| | width2 = | |||
| ⚫ | | tradename = | ||
| ⚫ | | alt2 |
||
| ⚫ | | Drugs.com = {{drugs.com|monograph|codeine}} | ||
| ⚫ | | caption |
||
| ⚫ | | MedlinePlus = a682065 | ||
| ⚫ | | pronounce |
||
| | DailyMedID = Codeine | |||
| ⚫ | | tradename |
||
| ⚫ | | pregnancy_AU = A | ||
| ⚫ | | Drugs.com |
||
| ⚫ | | MedlinePlus |
||
| | licence_CA = <!-- Health Canada may use generic or brand name (generic name preferred) --> | |||
| | licence_EU = <!-- EMA uses INN (or special INN_EMA) --> | |||
| | DailyMedID = <!-- DailyMed may use generic or brand name (generic name preferred) --> | |||
| | licence_US = <!-- FDA may use generic or brand name (generic name preferred) --> | |||
| ⚫ | | pregnancy_AU |
||
| | pregnancy_AU_comment = <ref name="Drugs.com pregnancy">{{cite web | title=Codeine Use During Pregnancy | website=Drugs.com | date=3 February 2020 | url=https://www.drugs.com/pregnancy/codeine.html | access-date=7 February 2020 | archive-date=30 December 2019 | archive-url=https://web.archive.org/web/20191230164005/https://www.drugs.com/pregnancy/codeine.html | url-status=live }}</ref> | | pregnancy_AU_comment = <ref name="Drugs.com pregnancy">{{cite web | title=Codeine Use During Pregnancy | website=Drugs.com | date=3 February 2020 | url=https://www.drugs.com/pregnancy/codeine.html | access-date=7 February 2020 | archive-date=30 December 2019 | archive-url=https://web.archive.org/web/20191230164005/https://www.drugs.com/pregnancy/codeine.html | url-status=live }}</ref> | ||
| | pregnancy_US = C | |||
| | pregnancy_US_comment = <ref name="Drugs.com pregnancy" /> | |||
| | pregnancy_category = | | pregnancy_category = | ||
| | dependency_liability = | | dependency_liability = High | ||
| | addiction_liability = High<ref>{{cite book |vauthors=Bonewit-West K, Hunt SA, Applegate E |title=Today's Medical Assistant: Clinical and Administrative Procedures |date=2012 |page=571 |publisher=Elsevier Health Sciences |isbn=9781455701506 |url=https://books.google.com/books?id=YalYPI1KqTQC&pg=PA571 |access-date=20 August 2019 |archive-date=10 January 2023 |archive-url=https://web.archive.org/web/20230110030031/https://books.google.com/books?id=YalYPI1KqTQC&pg=PA571 |url-status=live }}</ref> | | addiction_liability = High<ref>{{cite book |vauthors=Bonewit-West K, Hunt SA, Applegate E |title=Today's Medical Assistant: Clinical and Administrative Procedures |date=2012 |page=571 |publisher=Elsevier Health Sciences |isbn=9781455701506 |url=https://books.google.com/books?id=YalYPI1KqTQC&pg=PA571 |access-date=20 August 2019 |archive-date=10 January 2023 |archive-url=https://web.archive.org/web/20230110030031/https://books.google.com/books?id=YalYPI1KqTQC&pg=PA571 |url-status=live }}</ref> | ||
| | routes_of_administration = ], ], ], ] | | routes_of_administration = ], ], ], ] | ||
| | class |
| class = {{ubli|]|]}} | ||
| ⚫ | | ATC_prefix = R05 | ||
| | ATCvet = | |||
| ⚫ | | ATC_suffix = DA04 | ||
| ⚫ | | ATC_prefix |
||
| ⚫ | | ATC_supplemental = Combinations: {{ATC|N02|AA59}}, {{ATC|N02|AA79}}, {{ATC|N02|AJ08}}, {{ATC|N02|AJ06}}, {{ATC|N02|AJ07}} | ||
| ⚫ | | ATC_suffix |
||
| ⚫ | | ATC_supplemental |
||
| <!-- Legal status -->| legal_AU |
<!-- Legal status -->| legal_AU = S8 | ||
| | legal_AU_comment |
| legal_AU_comment = / S4 (Prescription only){{efn|S4 only if in drug combinations; see ].}} | ||
| | legal_BR |
| legal_BR = A2 | ||
| | legal_BR_comment |
| legal_BR_comment = / ]{{efn|Class C1 only for low doses; see exemptions on ].}} | ||
| | legal_CA |
| legal_CA = Schedule I | ||
| | legal_CA_comment |
| legal_CA_comment = | ||
| | legal_DE |
| legal_DE = Anlage III | ||
| | legal_DE_comment |
| legal_DE_comment = | ||
| | legal_NZ |
| legal_NZ = Class C | ||
| | legal_NZ_comment |
| legal_NZ_comment = | ||
| | legal_UK |
| legal_UK = Class B | ||
| | legal_UK_comment |
| legal_UK_comment = / ]{{efn|Pharmacy medicine if purchased in a low dose from a licensed pharmacy or in low dose drug combination; see ].}} | ||
| | legal_US |
| legal_US = Schedule II | ||
| | legal_US_comment |
| legal_US_comment = / Schedule III–V{{efn|Schedule III-V only if in drug combination; see ].}} | ||
| | legal_UN |
| legal_UN = N II | ||
| | legal_UN_comment |
| legal_UN_comment = / Narcotic Schedule III{{efn|Schedule III only if in drug combination; see ].}} | ||
| | legal_status |
| legal_status = <!-- For countries not listed above --> | ||
| <!-- Pharmacokinetic data -->| bioavailability = c. 60% (by mouth)<ref name="FiresteinBudd2016">{{cite book| vauthors= Polsten GR, Wallace MS | chapter = Analgesic Agents in Rheumatic Disease | veditors = Firestein GS, Budd R, Gabriel SE, McInnes IB, O'Dell JR |title=Kelley and Firestein's Textbook of Rheumatology | chapter-url = https://books.google.com/books?id=kBZ6DAAAQBAJ&pg=PA1081 |date=21 June 2016|publisher=Elsevier Health Sciences|isbn=978-0-323-41494-4|pages=1081–}}</ref> | |||
| <!-- Pharmacokinetic data -->| bioavailability = Oral: ~90% | |||
| | protein_bound |
| protein_bound = | ||
| | metabolism |
| metabolism = ]: ] (to ]), ] (to ]), ] (to 3- and 6-]s of codeine, norcodeine, and morphine)<ref name="pmid17470523">{{cite journal | vauthors = Shen H, He MM, Liu H, Wrighton SA, Wang L, Guo B, Li C | title = Comparative metabolic capabilities and inhibitory profiles of CYP2D6.1, CYP2D6.10, and CYP2D6.17 | journal = Drug Metabolism and Disposition | volume = 35 | issue = 8 | pages = 1292–1300 | date = August 2007 | pmid = 17470523 | doi = 10.1124/dmd.107.015354 | s2cid = 2322678 }}</ref> | ||
| | metabolites |
| metabolites = | ||
| *] | |||
| * ] | |||
| * Others (e.g., ]s) | |||
| | onset |
| onset = 15–30 minutes<ref name=AHFS2016 /> | ||
| | elimination_half-life = 2.5–3 hours | | elimination_half-life = 2.5–3 hours | ||
| | duration_of_action = 4–6 hours<ref name=AHFS2016 /> | | duration_of_action = 4–6 hours<ref name=AHFS2016 /> | ||
| | excretion |
| excretion = <!-- Identifiers --> | ||
| | CAS_number_Ref |
| CAS_number_Ref = {{cascite|correct|??}} | ||
| | CAS_number |
| CAS_number = 76-57-3 | ||
| | CAS_supplemental |
| CAS_supplemental = | ||
| | PubChem |
| PubChem = 5284371 | ||
| | IUPHAR_ligand |
| IUPHAR_ligand = 1673 | ||
| | DrugBank_Ref |
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| | DrugBank |
| DrugBank = DB00318 | ||
| | ChemSpiderID_Ref |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID |
| ChemSpiderID = 4447447 | ||
| | UNII_Ref |
| UNII_Ref = {{fdacite|changed|FDA}} | ||
| | UNII |
| UNII = UX6OWY2V7J | ||
| | KEGG_Ref |
| KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | KEGG |
| KEGG = C06174 | ||
| | KEGG2_Ref |
| KEGG2_Ref = {{keggcite|correct|kegg}} | ||
| | KEGG2 |
| KEGG2 = | ||
| | ChEBI_Ref |
| ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| | ChEBI |
| ChEBI = 16714 | ||
| | ChEMBL_Ref |
| ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| | ChEMBL |
| ChEMBL = 485 | ||
| | NIAID_ChemDB |
| NIAID_ChemDB = | ||
| | PDB_ligand |
| PDB_ligand = | ||
| | synonyms |
| synonyms = 3-Methylmorphine | ||
| <!-- Chemical and physical data -->| IUPAC_name |
<!-- Chemical and physical data -->| IUPAC_name = (5α,6α)-7,8-didehydro-4,5-epoxy-3-methoxy-17-methylmorphinan-6-ol | ||
| | C = 18 | |||
| | chemical_formula = | |||
| | H = 21 | |||
| | C = 18 | |||
| | N = 1 | |||
| | H = 21 | |||
| | |
| O = 3 | ||
| ⚫ | | SMILES = CN1CC2341CC5=C2C(=C(C=C5)OC)O3(C=C4)O | ||
| | O = 3 | |||
| ⚫ | | StdInChI_Ref = {{stdinchicite|changed|chemspider}} | ||
| ⚫ | | SMILES |
||
| ⚫ | | StdInChI = 1S/C18H21NO3/c1-19-8-7-18-11-4-5-13(20)17(18)22-16-14(21-2)6-3-10(15(16)18)9-12(11)19/h3-6,11-13,17,20H,7-9H2,1-2H3/t11-,12+,13-,17-,18-/m0/s1 | ||
| | Jmol = | |||
| ⚫ | | StdInChI_comment = | ||
| ⚫ | | StdInChI_Ref |
||
| ⚫ | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| ⚫ | | StdInChI |
||
| ⚫ | | StdInChIKey = OROGSEYTTFOCAN-DNJOTXNNSA-N | ||
| ⚫ | | StdInChI_comment |
||
| | density = | |||
| ⚫ | | StdInChIKey_Ref |
||
| | density_notes = | |||
| ⚫ | | StdInChIKey |
||
| | |
| melting_point = | ||
| | |
| melting_high = | ||
| | |
| melting_notes = | ||
| | |
| boiling_point = | ||
| | |
| boiling_notes = | ||
| | |
| solubility = | ||
| | |
| sol_units = | ||
| | |
| specific_rotation = | ||
| | sol_units = | |||
| | specific_rotation = | |||
| }} | }} | ||
| <!-- Definition and medical uses --> | <!-- Definition and medical uses --> | ||
| '''Codeine''' is an ] and ] of ] mainly used to treat ], ], and ]. It is also commonly used as a ]. It is found naturally in the sap of the ], ''Papaver somniferum''.<ref name=AHFS2016>{{cite web|title=Codeine|url=https://www.drugs.com/monograph/codeine.html|publisher=The American Society of Health-System Pharmacists|access-date=5 January 2016|url-status=live|archive-url=https://web.archive.org/web/20160118164241/http://www.drugs.com/monograph/codeine.html|archive-date=18 January 2016}}</ref><ref>{{cite journal | vauthors = Prommer E | title = Role of codeine in palliative care | journal = Journal of Opioid Management | volume = 7 | issue = 5 | pages = 401–406 | date = 2010 | pmid = 22165039 | doi = 10.5055/jom.2011.0081 }}</ref> It is typically used to treat mild to moderate degrees of pain.<ref name=AHFS2016 /> Greater benefit may occur ] with ] (acetaminophen) or a ] (NSAID) such as ] or ].<ref name=AHFS2016 /> Evidence does not support its use for acute cough suppression in children |
'''Codeine''' is an ] and ] of ] mainly used to treat ], ], and ]. It is also commonly used as a ]. It is found naturally in the sap of the ], ''Papaver somniferum''.<ref name=AHFS2016>{{cite web|title=Codeine |url=https://www.drugs.com/monograph/codeine.html |publisher=The American Society of Health-System Pharmacists |access-date=5 January 2016|url-status=live|archive-url=https://web.archive.org/web/20160118164241/http://www.drugs.com/monograph/codeine.html|archive-date=18 January 2016}}</ref><ref>{{cite journal | vauthors = Prommer E | title = Role of codeine in palliative care | journal = Journal of Opioid Management | volume = 7 | issue = 5 | pages = 401–406 | date = 2010 | pmid = 22165039 | doi = 10.5055/jom.2011.0081 }}</ref> It is typically used to treat mild to moderate degrees of pain.<ref name=AHFS2016 />{{Failed verification |date=March 2024 |reason=Recommended for that use in one country, nothing said about typical usage.}} Greater benefit may occur ] with ] (acetaminophen) or a ] (NSAID) such as ] or ].<ref name=AHFS2016 /> Evidence does not support its use for acute cough suppression in children.<ref name="paul-im-2012">{{cite journal | vauthors = Paul IM | title = Therapeutic options for acute cough due to upper respiratory infections in children | journal = Lung | volume = 190 | issue = 1 | pages = 41–44 | date = February 2012 | pmid = 21892785 | doi = 10.1007/s00408-011-9319-y | s2cid = 23865647 }}</ref><ref>{{cite journal |vauthors=Smith SM, Schroeder K, Fahey T |date=November 2014 |title=Over-the-counter (OTC) medications for acute cough in children and adults in community settings |journal=The Cochrane Database of Systematic Reviews |volume=2014 |issue= 11|pages= CD001831|doi=10.1002/14651858.CD001831.pub5 |pmid=25420096|pmc=7061814 }}</ref> In Europe, it is not recommended as a cough medicine for those under 12 years of age.<ref name=AHFS2016 /> It is generally taken by mouth.<ref name=AHFS2016 /> It typically starts working after half an hour, with maximum effect at two hours.<ref name=AHFS2016 /> Its effects last for about four to six hours. Codeine exhibits abuse potential similar to other opioid medications, including a risk of ] and ].<ref name=AHFS2016 /> | ||
| <!-- Side effects and mechanism --> | <!-- Side effects and mechanism --> | ||
| Line 130: | Line 123: | ||
| ===Cough=== | ===Cough=== | ||
| Codeine is used to relieve ]ing.<ref name=AHFS2016/> Evidence does not support its use for acute cough suppression in children.<ref name="paul-im-2012" /> In Europe, it is not recommended as a cough medicine |
Codeine is used to relieve ]ing.<ref name=AHFS2016/> Evidence does not support its use for acute cough suppression in children.<ref name="paul-im-2012" /> In Europe, it is not recommended as a cough medicine for those under 12 years of age.<ref name=AHFS2016 /> Some tentative evidence shows it can reduce a chronic cough in adults.<ref>{{cite journal | vauthors = McCrory DC, Coeytaux RR, Yancy WS Jr, Schmit KM, Kemper AR, Goode A, Hasselblad V, Heidenfelder BL, Irvine RJ, Musty MD, Gray R, Sanders GD | title = Assessment and Management of Chronic Cough | journal = AHRQ Comparative Effectiveness Reviews | date = January 2013 | pmid = 23367526 }}</ref> | ||
| ===Diarrhea=== | ===Diarrhea=== | ||
| Line 137: | Line 130: | ||
| ===Formulations=== | ===Formulations=== | ||
| {{Unreferenced section|date=January 2023}} | {{Unreferenced section|date=January 2023}} | ||
| Codeine is marketed as both a single-ingredient drug and in combination preparations with paracetamol (as ]: |
Codeine is marketed as both a single-ingredient drug and in combination preparations with paracetamol (as ]: e.g., brands Paracod, Panadeine, and the Tylenol-with-codeine series, including ] and 1, 2, and 4); with ] (as ]); or with ] (as ]). These combinations provide greater pain relief than either agent alone (]). | ||
| Codeine is also commonly marketed in products containing codeine with other pain killers or muscle relaxers, as well as codeine mixed with ] (Emprazil with codeine No. 1, 2, 3, 4, and 5), ], ], ], and others, as well as more complex mixtures, including such mixtures as aspirin + paracetamol + codeine ± caffeine ± antihistamines and other agents, such as those mentioned above. | Codeine is also commonly marketed in products containing codeine with other pain killers or muscle relaxers, as well as codeine mixed with ] (Emprazil with codeine No. 1, 2, 3, 4, and 5), ], ], ], and others, as well as more complex mixtures, including such mixtures as aspirin + paracetamol + codeine ± caffeine ± antihistamines and other agents, such as those mentioned above. | ||
| Codeine-only products can be obtained with a prescription as a ]. Codeine is also marketed in cough syrups with zero to a half-dozen other active ingredients, and a ] ( |
Codeine-only products can be obtained with a prescription as a ]. Codeine is also marketed in cough syrups with zero to a half-dozen other active ingredients, and a ] (e.g., Paveral) for all of the uses for which codeine is indicated. | ||
| Injectable codeine is available for subcutaneous or intramuscular injection only; intravenous injection is contraindicated, as this can result in nonimmune mast-cell degranulation and resulting ] reaction. Codeine suppositories are also marketed in some countries. | Injectable codeine is available for subcutaneous or intramuscular injection only; intravenous injection is contraindicated, as this can result in nonimmune mast-cell degranulation and resulting ] reaction. Codeine suppositories are also marketed in some countries. | ||
| ==Side effects== | ==Side effects== | ||
| Common adverse effects associated with the use of codeine include ] and ]. Less common are ], ], ], ], ], ], ], ], and ]. Rare adverse effects include ], ], ], and ].<ref>{{cite web|publisher=WebMD LLC.|title=Codeine – adverse effects|url=http://reference.medscape.com/drug/codeine-343310#4|work=Medscape reference – Drugs, Diseases & Procedures|access-date=27 September 2012|url-status=live|archive-url=https://web.archive.org/web/20120818214647/http://reference.medscape.com/drug/codeine-343310#4|archive-date=18 August 2012}}</ref> As with all opiates, long-term effects can vary, but can include diminished libido, apathy, and memory loss. Some people may have allergic reactions to codeine, such as the swelling of skin and rashes.<ref name=AHFS2016/> | Common adverse effects associated with the use of codeine include ] and ]. Less common are ], ], ], ], ], ], ], ], and ]. Rare adverse effects include ], ], ], and ].<ref>{{cite web|publisher=WebMD LLC.|title=Codeine – adverse effects|url=http://reference.medscape.com/drug/codeine-343310#4|work=Medscape reference – Drugs, Diseases & Procedures|access-date=27 September 2012|url-status=live|archive-url=https://web.archive.org/web/20120818214647/http://reference.medscape.com/drug/codeine-343310#4|archive-date=18 August 2012}}</ref> As with all opiates, long-term effects can vary, but can include diminished libido, apathy, and memory loss. Some people may have allergic reactions to codeine, such as the swelling of the skin and rashes.<ref name=AHFS2016/> | ||
| Tolerance to many of the effects of codeine, including its therapeutic effects, develops with prolonged use. This occurs at different rates for different effects, with tolerance to the constipation-inducing effects developing particularly slowly for instance. | Tolerance to many of the effects of codeine, including its therapeutic effects, develops with prolonged use. This occurs at different rates for different effects, with tolerance to the constipation-inducing effects developing particularly slowly for instance. | ||
| Line 156: | Line 149: | ||
| ===Withdrawal and dependence=== | ===Withdrawal and dependence=== | ||
| As with other opiates, chronic use of codeine can cause ] which can lead to severe ] if a person suddenly stops the medication. Withdrawal symptoms include drug craving, runny nose, yawning, sweating, insomnia, weakness, stomach cramps, nausea, vomiting, diarrhea, muscle spasms, chills, irritability, and pain. These side |
As with other opiates, chronic use of codeine can cause ] which can lead to severe ] if a person suddenly stops the medication. Withdrawal symptoms include drug craving, runny nose, yawning, sweating, insomnia, weakness, stomach cramps, nausea, vomiting, diarrhea, muscle spasms, chills, irritability, and pain. These side effects also occur in acetaminophen/aspirin combinations, though to a lesser extent. To minimize withdrawal symptoms, long-term users should gradually reduce their codeine medication under the supervision of a healthcare professional.<ref>{{cite web|title=Opioids: Information for Health Professionals|url=http://www.albertahealthservices.ca/assets/info/res/mhr/if-res-mhr-hp-opioid-info.pdf|publisher=Alberta Health Services|access-date=9 March 2017|url-status=live|archive-url=https://web.archive.org/web/20170312032436/http://www.albertahealthservices.ca/assets/info/res/mhr/if-res-mhr-hp-opioid-info.pdf|archive-date=12 March 2017}}</ref> | ||
| Also, no evidence indicates that ] inhibition is useful in treating codeine dependence,<ref>{{cite journal | vauthors = Fernandes LC, Kilicarslan T, Kaplan HL, Tyndale RF, Sellers EM, Romach MK | title = Treatment of codeine dependence with inhibitors of cytochrome P450 2D6 | journal = Journal of Clinical Psychopharmacology | volume = 22 | issue = 3 | pages = 326–329 | date = June 2002 | pmid = 12006904 | doi = 10.1097/00004714-200206000-00014 | s2cid = 30804655 }}</ref> though the metabolism of codeine to morphine (and hence further metabolism to glucuronide morphine conjugates) does have an effect on the abuse potential of codeine.<ref>{{cite journal | vauthors = Kathiramalainathan K, Kaplan HL, Romach MK, Busto UE, Li NY, Säwe J, Tyndale RF, Sellers EM |
Also, no evidence indicates that ] inhibition is useful in treating codeine dependence,<ref>{{cite journal | vauthors = Fernandes LC, Kilicarslan T, Kaplan HL, Tyndale RF, Sellers EM, Romach MK | title = Treatment of codeine dependence with inhibitors of cytochrome P450 2D6 | journal = Journal of Clinical Psychopharmacology | volume = 22 | issue = 3 | pages = 326–329 | date = June 2002 | pmid = 12006904 | doi = 10.1097/00004714-200206000-00014 | s2cid = 30804655 }}</ref> though the metabolism of codeine to morphine (and hence further metabolism to glucuronide morphine conjugates) does have an effect on the abuse potential of codeine.<ref>{{cite journal | vauthors = Kathiramalainathan K, Kaplan HL, Romach MK, Busto UE, Li NY, Säwe J, Tyndale RF, Sellers EM | title = Inhibition of cytochrome P450 2D6 modifies codeine abuse liability | journal = Journal of Clinical Psychopharmacology | volume = 20 | issue = 4 | pages = 435–444 | date = August 2000 | pmid = 10917405 | doi = 10.1097/00004714-200008000-00008 }}</ref> However, CYP2D6 has been implicated in the toxicity and death of neonates when codeine is administered to lactating mothers, particularly those with increased enzyme activity ("ultra-rapid" metabolizers).<ref name="koren etal" /><ref>{{cite journal | vauthors = Willmann S, Edginton AN, Coboeken K, Ahr G, Lippert J | title = Risk to the breast-fed neonate from codeine treatment to the mother: a quantitative mechanistic modeling study | journal = Clinical Pharmacology and Therapeutics | volume = 86 | issue = 6 | pages = 634–643 | date = December 2009 | pmid = 19710640 | doi = 10.1038/clpt.2009.151 | s2cid = 37771918 }}</ref> | ||
| In 2019 Ireland was said to be on the verge of a codeine addiction epidemic, according to a paper in the ]. Under Irish law, codeine can be bought over |
In 2019 Ireland was said to be on the verge of a codeine addiction epidemic, according to a paper in the ]. Under Irish law, codeine can be bought over the counter under the supervision of a pharmacist, but there is no mechanism to detect patients travelling to different pharmacies to purchase codeine.<ref>{{cite news |title=Ireland on the verge of a codeine addiction epidemic, Irish Medical Journal warns |url=https://www.irishexaminer.com/news/arid-30911037.html |access-date=16 November 2022 |publisher=Irish Examiner |date=14 March 2019 |archive-date=16 November 2022 |archive-url=https://web.archive.org/web/20221116102257/https://www.irishexaminer.com/news/arid-30911037.html |url-status=live }}</ref> | ||
| ==Pharmacology== | ==Pharmacology== | ||
| Line 205: | Line 198: | ||
| Codeine is a nonsynthetic ].<ref name="Moore2012">{{cite book | vauthors = Szucs-Reed RP, Gallagher RM | chapter = Chronic pain and opioids. | veditors = Moore RJ |title=Handbook of Pain and Palliative Care: Biobehavioral Approaches for the Life Course| chapter-url=https://books.google.com/books?id=bpvcJiCQb1wC&pg=PA499|date=5 January 2012|publisher=Springer Science & Business Media|isbn=978-1-4419-1651-8|pages=499– | doi = 10.1007/978-1-4419-1651-8_29 | s2cid = 68670125 }}</ref> It is a ] ] of the ] (MOR).<ref name="Moore2012" /> Codeine itself has relatively weak ] for the MOR.<ref name="Moore2012" /><ref name="pmid8114680" /> Instead of acting directly on the MOR, codeine functions as a ] of its major ]s morphine and ], which are far more ] MOR agonists in comparison.<ref name="Moore2012" /><ref name="CorbettPaterson1993" /> | Codeine is a nonsynthetic ].<ref name="Moore2012">{{cite book | vauthors = Szucs-Reed RP, Gallagher RM | chapter = Chronic pain and opioids. | veditors = Moore RJ |title=Handbook of Pain and Palliative Care: Biobehavioral Approaches for the Life Course| chapter-url=https://books.google.com/books?id=bpvcJiCQb1wC&pg=PA499|date=5 January 2012|publisher=Springer Science & Business Media|isbn=978-1-4419-1651-8|pages=499– | doi = 10.1007/978-1-4419-1651-8_29 | s2cid = 68670125 }}</ref> It is a ] ] of the ] (MOR).<ref name="Moore2012" /> Codeine itself has relatively weak ] for the MOR.<ref name="Moore2012" /><ref name="pmid8114680" /> Instead of acting directly on the MOR, codeine functions as a ] of its major ]s morphine and ], which are far more ] MOR agonists in comparison.<ref name="Moore2012" /><ref name="CorbettPaterson1993" /> | ||
| Codeine has been found as an endogenous compound, along with morphine, in the brains of nonhuman primates with depolarized neurons, indicating that codeine may function as a neurotransmitter or neuromodulator in the central nervous system.<ref>Neri C; Guama M; et al. Endogenous morphine and codeine in the brain of non human primate. Medical Science Montier 2004, 10, MS1-MS5.</ref> Like morphine, codeine causes TLR4 signaling which causes ] and ].<ref name="pmid25386959">{{cite journal | vauthors = Johnson JL, Rolan PE, Johnson ME, Bobrovskaya L, Williams DB, Johnson K, Tuke J, Hutchinson MR |
Codeine has been found as an endogenous compound, along with morphine, in the brains of nonhuman primates with depolarized neurons, indicating that codeine may function as a neurotransmitter or neuromodulator in the central nervous system.<ref>Neri C; Guama M; et al. Endogenous morphine and codeine in the brain of non human primate. Medical Science Montier 2004, 10, MS1-MS5.</ref> Like morphine, codeine causes TLR4 signaling which causes ] and ].<ref name="pmid25386959">{{cite journal | vauthors = Johnson JL, Rolan PE, Johnson ME, Bobrovskaya L, Williams DB, Johnson K, Tuke J, Hutchinson MR | title = Codeine-induced hyperalgesia and allodynia: investigating the role of glial activation | journal = Translational Psychiatry | volume = 4 | issue = 11 | pages = e482 | date = November 2014 | pmid = 25386959 | pmc = 4259992 | doi = 10.1038/tp.2014.121 }}</ref> It does not need to be converted to morphine to increase pain sensitivity.<ref name="pmid25386959"/> | ||
| ===Mechanism of action=== | ===Mechanism of action=== | ||
| Codeine is an |
Codeine is an opiate and an agonist of the ] (MOR). It acts on the central nervous system to have an analgesic effect.<ref name="Codeine">{{cite book | vauthors = Papich MG | chapter = Codeine | title = Saunders Handbook of Veterinary Drugs | edition = fourth |date=1 January 2016 |pages=183–184 |doi=10.1016/b978-0-323-24485-5.00175-3 |publisher=W.B. Saunders|isbn=978-0-323-24485-5 }}</ref> It is metabolised in the liver to produce morphine which is ten times more potent against the mu receptor. Opioid receptors are ] that positively and negatively regulate synaptic transmission through downstream signalling. Binding of codeine or morphine to the mu-opioid receptor results in hyperpolarization of the neuron leading to the inhibition of the release of nociceptive neurotransmitters, causing an analgesic effect and increased pain tolerance due to reduced neuronal excitability.<ref name="Codeine"/><ref>{{cite book | vauthors = Hitchings A, Lonsdale D, Burrage D, Baker E |title=Top 100 drugs: clinical pharmacology and practical prescribing |date=2015 |isbn=978-0-7020-5516-4 |page=168 |publisher=Churchill Livingstone }}</ref> | ||
| ===Pharmacokinetics=== | ===Pharmacokinetics=== | ||
| The conversion of codeine to morphine occurs in the liver and is catalyzed by the ] enzyme ].<ref name="St2012" /> ] produces ], and ] conjugates codeine, norcodeine, and morphine to the corresponding 3- and 6-glucuronides. Srinivasan, Wielbo and Tebbett speculate that codeine-6-glucuronide is responsible for a large percentage of the analgesia of codeine, and thus these patients should experience some analgesia.<ref name="Srinivasan V, Wielbo D, Tebbett IR 1997 185–90">{{cite journal | vauthors = Srinivasan V, Wielbo D, Tebbett IR | title = Analgesic effects of codeine-6-glucuronide after intravenous administration | journal = European Journal of Pain | volume = 1 | issue = 3 | pages = 185–190 | year = 1997 | pmid = 15102399 | doi = 10.1016/S1090-3801(97)90103-8 | s2cid = 23099329 }}</ref> Many of the adverse effects will still be experienced in poor metabolizers. Conversely, between 0.5% and 2% of the population are "extensive metabolizers"; multiple copies of the gene for 2D6 produce high levels of CYP2D6 and will metabolize drugs through that pathway more quickly than others. | The conversion of codeine to morphine occurs in the liver and is catalyzed by the ] enzyme ].<ref name="St2012" /> ] produces ], and ] conjugates codeine, norcodeine, and morphine to the corresponding 3- and 6-glucuronides. Srinivasan, Wielbo, and Tebbett speculate that codeine-6-glucuronide is responsible for a large percentage of the analgesia of codeine, and thus these patients should experience some analgesia.<ref name="Srinivasan V, Wielbo D, Tebbett IR 1997 185–90">{{cite journal | vauthors = Srinivasan V, Wielbo D, Tebbett IR | title = Analgesic effects of codeine-6-glucuronide after intravenous administration | journal = European Journal of Pain | volume = 1 | issue = 3 | pages = 185–190 | year = 1997 | pmid = 15102399 | doi = 10.1016/S1090-3801(97)90103-8 | s2cid = 23099329 }}</ref> Many of the adverse effects will still be experienced in poor metabolizers. Conversely, between 0.5% and 2% of the population are "extensive metabolizers"; multiple copies of the gene for 2D6 produce high levels of CYP2D6 and will metabolize drugs through that pathway more quickly than others. | ||
| Some medications are CYP2D6 inhibitors and reduce or even completely block the conversion of codeine to morphine. The best-known of these are two of the ]s, ] (Paxil) and ] (Prozac) as well as the antihistamine ] (Benadryl) and the antidepressant ] (Wellbutrin, also known as Zyban). Other drugs, such as ] and ], induce CYP450 isozymes and thus increase the conversion rate. | Some medications are CYP2D6 inhibitors and reduce or even completely block the conversion of codeine to morphine. The best-known of these are two of the ]s, ] (Paxil) and ] (Prozac) as well as the antihistamine ] (Benadryl) and the antidepressant ] (Wellbutrin, also known as Zyban). Other drugs, such as ] and ], induce CYP450 isozymes and thus increase the conversion rate. | ||
| Line 220: | Line 213: | ||
| Studies on codeine's analgesic effect are consistent with the idea that metabolism by CYP2D6 to morphine is important, but some studies show no major differences between those who are poor metabolizers and extensive metabolizers. Evidence supporting the hypothesis that ultrarapid metabolizers may get greater analgesia from codeine due to increased morphine formation is limited to case reports.<ref>{{cite journal | vauthors = Gardiner SJ, Begg EJ | title = Pharmacogenetics, drug-metabolizing enzymes, and clinical practice | journal = Pharmacological Reviews | volume = 58 | issue = 3 | pages = 521–590 | date = September 2006 | pmid = 16968950 | doi = 10.1124/pr.58.3.6 | s2cid = 25747320 }}</ref> | Studies on codeine's analgesic effect are consistent with the idea that metabolism by CYP2D6 to morphine is important, but some studies show no major differences between those who are poor metabolizers and extensive metabolizers. Evidence supporting the hypothesis that ultrarapid metabolizers may get greater analgesia from codeine due to increased morphine formation is limited to case reports.<ref>{{cite journal | vauthors = Gardiner SJ, Begg EJ | title = Pharmacogenetics, drug-metabolizing enzymes, and clinical practice | journal = Pharmacological Reviews | volume = 58 | issue = 3 | pages = 521–590 | date = September 2006 | pmid = 16968950 | doi = 10.1124/pr.58.3.6 | s2cid = 25747320 }}</ref> | ||
| Due to increased metabolism of codeine to morphine, ultrarapid metabolizers (those possessing more than two functional copies of the CYP2D6 allele) are at increased risk of adverse drug effects related to morphine toxicity. Guidelines released by the Clinical Pharmacogenomics Implementation Consortium (CPIC) advise against administering codeine to ultrarapid metabolizers, where this genetic information is available. The CPIC also suggests that codeine use be avoided in poor metabolizers, due to its lack of efficacy in this group.<ref>{{cite journal | vauthors = Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE, Caudle KE, Haidar CE, Shen DD, Callaghan JT, Sadhasivam S, Prows CA, Kharasch ED, Skaar TC |
Due to the increased metabolism of codeine to morphine, ultrarapid metabolizers (those possessing more than two functional copies of the CYP2D6 allele) are at increased risk of adverse drug effects related to morphine toxicity. Guidelines released by the Clinical Pharmacogenomics Implementation Consortium (CPIC) advise against administering codeine to ultrarapid metabolizers, where this genetic information is available. The CPIC also suggests that codeine use be avoided in poor metabolizers, due to its lack of efficacy in this group.<ref>{{cite journal | vauthors = Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE, Caudle KE, Haidar CE, Shen DD, Callaghan JT, Sadhasivam S, Prows CA, Kharasch ED, Skaar TC | title = Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update | journal = Clinical Pharmacology and Therapeutics | volume = 95 | issue = 4 | pages = 376–382 | date = April 2014 | pmid = 24458010 | pmc = 3975212 | doi = 10.1038/clpt.2013.254 }}</ref> | ||
| Codeine and its salts are readily absorbed from the gastrointestinal tract, and ingestion of codeine phosphate produces peak plasma concentrations in about one hour. Plasma half life is between 3 and 4 hours, and oral/intramuscular analgesic potency ratio is approximately equal to 1:1.5. The most common conversion ratio, given on equianalgesia charts used in the United States, Canada, the UK, Republic of Ireland, the European Union, Russia and elsewhere as 130 mg IM equals 200 mg PO—both of which are equivalent to 10 mg of morphine sulphate IV and 60 mg of morphine sulphate PO. The salt:freebase ratio of the salts of both drugs in use are roughly equivalent, and do not generally make a clinical difference.<ref>The Merck Index, 13th Edition: Morphine Hydrochloride</ref> | Codeine and its salts are readily absorbed from the gastrointestinal tract, and ingestion of codeine phosphate produces peak plasma concentrations in about one hour. Plasma half life is between 3 and 4 hours, and oral/intramuscular analgesic potency ratio is approximately equal to 1:1.5. The most common conversion ratio, given on equianalgesia charts used in the United States, Canada, the UK, Republic of Ireland, the European Union, Russia and elsewhere as 130 mg IM equals 200 mg PO—both of which are equivalent to 10 mg of morphine sulphate IV and 60 mg of morphine sulphate PO. The salt:freebase ratio of the salts of both drugs in use are roughly equivalent, and do not generally make a clinical difference.<ref>The Merck Index, 13th Edition: Morphine Hydrochloride</ref> | ||
| Line 226: | Line 219: | ||
| Codeine is metabolised by ''O''- and ''N''-demethylation in the liver to morphine and norcodeine. ] is also a metabolite of codeine in humans.<ref name="pmid37301">{{cite journal | vauthors = Cone EJ, Darwin WD, Gorodetzky CW | title = Comparative metabolism of codeine in man, rat, dog, guinea-pig and rabbit: identification of four new metabolites | journal = The Journal of Pharmacy and Pharmacology | volume = 31 | issue = 5 | pages = 314–317 | date = May 1979 | pmid = 37301 | doi = 10.1111/j.2042-7158.1979.tb13507.x | s2cid = 42128300 }}</ref> Codeine and its metabolites are mostly removed from the body by the kidneys, primarily as conjugates with glucuronic acid.<ref>{{cite web |title=Codeine Phosphate Tablets 30mg - Summary of Product Characteristics (SmPC) - (emc) |url=http://www.medicines.org.uk/emc/medicine/23910#PHARMACOKINETIC_PROPS |url-status=dead |archive-url=https://web.archive.org/web/20220123193235/https://www.medicines.org.uk/emc/medicine/23910 |archive-date=23 January 2022 |access-date=29 December 2019 |website=www.medicines.org.uk}}</ref> | Codeine is metabolised by ''O''- and ''N''-demethylation in the liver to morphine and norcodeine. ] is also a metabolite of codeine in humans.<ref name="pmid37301">{{cite journal | vauthors = Cone EJ, Darwin WD, Gorodetzky CW | title = Comparative metabolism of codeine in man, rat, dog, guinea-pig and rabbit: identification of four new metabolites | journal = The Journal of Pharmacy and Pharmacology | volume = 31 | issue = 5 | pages = 314–317 | date = May 1979 | pmid = 37301 | doi = 10.1111/j.2042-7158.1979.tb13507.x | s2cid = 42128300 }}</ref> Codeine and its metabolites are mostly removed from the body by the kidneys, primarily as conjugates with glucuronic acid.<ref>{{cite web |title=Codeine Phosphate Tablets 30mg - Summary of Product Characteristics (SmPC) - (emc) |url=http://www.medicines.org.uk/emc/medicine/23910#PHARMACOKINETIC_PROPS |url-status=dead |archive-url=https://web.archive.org/web/20220123193235/https://www.medicines.org.uk/emc/medicine/23910 |archive-date=23 January 2022 |access-date=29 December 2019 |website=www.medicines.org.uk}}</ref> | ||
| The active metabolites of codeine, notably morphine, exert their effects by binding to and activating the ]. In people that can extensively metabolize the codeine, a 30 mg dose could |
The active metabolites of codeine, notably morphine, exert their effects by binding to and activating the ]. In people that can extensively metabolize the codeine, a 30 mg dose could yield up to 4 mg of morphine.<ref>{{cite web | url=https://medsafe.govt.nz/profs/PUArticles/June2018/SpotlightOnCodeine.htm#:~:text=Extensive%20metabolisers%20convert%205%E2%80%9315,to%204.5%20mg%20of%20morphine | title=Spotlight on Codeine }}</ref> | ||
| ==Chemistry== | ==Chemistry== | ||
| While codeine can be directly extracted from opium, its |
While codeine can be directly extracted from opium, its source, most codeine is synthesized from the much more abundant ] through the process of O-],<ref name="sciencedaily" /><ref>{{cite web |title=Virtual ChemBook |url=http://www.elmhurst.edu/~chm/vchembook/674narcotic.html |website=chemistry.elmhurst.edu |access-date=29 December 2019 |archive-date=29 July 2003 |archive-url=https://web.archive.org/web/20030729104518/http://www.elmhurst.edu/~chm/vchembook/674narcotic.html |url-status=dead }}</ref> through a process first completed in the late 20th century by Robert C. Corcoran and Junning Ma.<ref>{{cite patent | country = US | number = 6204337 | invent1 = Robert C. Corcoran | invent2 = Junning Ma | status = expired | ||
| | title = Solid-phase synthesis of codeine from morphine | pubdate = 2001-03-20 | gdate = 2001-03-20 | fdate = 1999-09-03 | pridate = 1999-09-03 | assign1 = University and Community College System of Nevada UCCSN }}</ref> | |||
| ===Relation to other opioids=== | ===Relation to other opioids=== | ||
| Codeine has been used in the past as the starting material and prototype of a large class of mainly mild to moderately strong opioids |
Codeine has been used in the past as the starting material and prototype of a large class of mainly mild to moderately strong opioids, such as ] (1920 in Germany), ] (1916 in Germany), ] (1908 in Germany), and its derivatives such as ] (1956 in Austria).{{Citation needed|date=May 2013}} However, these ] are no longer synthesized from codeine and are usually synthesized from other ] alkaloids, specifically ].<ref>{{Cite news |title=How Johnson & Johnson companies used a 'super poppy' to make narcotics for America's most abused opioid pills |url=https://www.washingtonpost.com/graphics/2020/business/opioid-crisis-johnson-and-johnson-tasmania-poppy/ |access-date=20 April 2022 |newspaper=The Washington Post |language=en |archive-date=15 May 2022 |archive-url=https://web.archive.org/web/20220515213704/https://www.washingtonpost.com/graphics/2020/business/opioid-crisis-johnson-and-johnson-tasmania-poppy/ |url-status=live }}</ref> Other series of codeine derivatives include ] and its derivatives, which were developed in Germany starting around 1920. In general, the various classes of morphine derivatives such as ketones, semisynthetics like ], halogeno-morphides, esters, ethers, and others have codeine, dihydrocodeine, and isocodeine analogues.<ref>Report of Committee on drug addiction, 1929–1941. National Research Council (US).</ref> The codeine ester ] is a common active impurity in street ] as some codeine tends to dissolve with the morphine when it is extracted from ] in underground heroin and morphine base labs. | ||
| As an analgesic, codeine ]. Related to codeine in other ways are ], ], ] (genocodeine), related to the nitrogen morphine derivatives as is codeine methobromide, and ], which is a drug six times stronger than morphine and 72 times stronger than codeine due to a small re-arrangement of the molecule, namely moving the methyl group from the 3 to the 6 position on the morphine carbon skeleton. | As an analgesic, codeine ]. Related to codeine in other ways are ], ], ] (genocodeine), related to the nitrogen morphine derivatives as is codeine methobromide, and ], which is a drug six times stronger than morphine and 72 times stronger than codeine due to a small re-arrangement of the molecule, namely moving the methyl group from the 3 to the 6 position on the morphine carbon skeleton. | ||
| Line 244: | Line 238: | ||
| Codeine is found in concentrations of 1% to 3% in opium prepared by the latex method from unripe pods of ''Papaver somniferum''. The name codeine is derived from the ] {{lang|grc|κώδεια}} ({{lang|grc-Latn|kṓdeia}}, "poppy head"). The relative proportion of codeine to morphine, the most common opium alkaloid at 4% to 23%, tends to be somewhat higher in the ] method of preparing opium alkaloids. | Codeine is found in concentrations of 1% to 3% in opium prepared by the latex method from unripe pods of ''Papaver somniferum''. The name codeine is derived from the ] {{lang|grc|κώδεια}} ({{lang|grc-Latn|kṓdeia}}, "poppy head"). The relative proportion of codeine to morphine, the most common opium alkaloid at 4% to 23%, tends to be somewhat higher in the ] method of preparing opium alkaloids. | ||
| Until the beginning of the 19th century, raw opium was used in diverse preparations known as ] (see ]'s '']'', 1821) and ] ]s, |
Until the beginning of the 19th century, raw opium was used in diverse preparations known as ] (see ]'s '']'', 1821) and ] ]s, several which were popular in England since the beginning of the 18th century; the original preparation seems to have been elaborated in ], the ] around 1715 by a chemist Jakob Le Mort; in 1721 the ''London Pharmacopoeia'' mentions an Elixir Asthmaticum, replaced by the term Elixir Paregoricum ("pain soother") in 1746. | ||
| The progressive isolation of opium's several active components opened the path to improved selectivity and safety of the opiates-based pharmacopeia. | The progressive isolation of opium's several active components opened the path to improved selectivity and safety of the opiates-based pharmacopeia. | ||
| Line 259: | Line 253: | ||
| ===Names=== | ===Names=== | ||
| It is often sold as a salt in the form of either codeine sulfate or codeine phosphate in the United States, United Kingdom and Australia. Codeine hydrochloride is more common worldwide and the citrate, hydroiodide, hydrobromide, tartrate, and other salts are also seen.<ref>Merck Index</ref> The chemical name for codeine is morphinan-6-ol, 7,8-didehydro-4,5-epoxy-3-methoxy-17-methyl-, (5α,6α)-<ref>Anonymous Codeine. In The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals; O'Neil, M. J., Ed.; Merck: Whitehouse Station, NJ, 2006; pp 2417.</ref> | It is often sold as a salt in the form of either codeine sulfate or codeine phosphate in the United States, United Kingdom, and Australia. Codeine hydrochloride is more common worldwide and the citrate, hydroiodide, hydrobromide, tartrate, and other salts are also seen.<ref>Merck Index</ref> The chemical name for codeine is morphinan-6-ol, 7,8-didehydro-4,5-epoxy-3-methoxy-17-methyl-, (5α,6α)-<ref>Anonymous Codeine. In The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals; O'Neil, M. J., Ed.; Merck: Whitehouse Station, NJ, 2006; pp 2417.</ref> | ||
| ===Recreational use=== | ===Recreational use=== | ||
| Line 265: | Line 259: | ||
| A ] (diamorphine) or other ]/] addict may use codeine to ward off the effects of ] during periods where their preferred drug is unavailable or unaffordable.<ref>{{Cite book | vauthors = van Solinge TB | title = L'héroïne, la cocaïne et le crack en France. Trafic, usage et politique | publisher = CEDRO Centrum voor Drugsonderzoek, Universiteit van Amsterdam | location = Amsterdam | language = fr | pages = 247–262 | chapter = 7. La politique de soins des années quatre-vingt-dix | chapter-url = http://www.cedro-uva.org/lib/boekhout.heroine.fr.7.html | year = 1996 | access-date = 26 April 2006 | archive-date = 25 February 2021 | archive-url = https://web.archive.org/web/20210225064644/http://www.cedro-uva.org/lib/boekhout.heroine.fr.7.html | url-status = live }}</ref> | A ] (diamorphine) or other ]/] addict may use codeine to ward off the effects of ] during periods where their preferred drug is unavailable or unaffordable.<ref>{{Cite book | vauthors = van Solinge TB | title = L'héroïne, la cocaïne et le crack en France. Trafic, usage et politique | publisher = CEDRO Centrum voor Drugsonderzoek, Universiteit van Amsterdam | location = Amsterdam | language = fr | pages = 247–262 | chapter = 7. La politique de soins des années quatre-vingt-dix | chapter-url = http://www.cedro-uva.org/lib/boekhout.heroine.fr.7.html | year = 1996 | access-date = 26 April 2006 | archive-date = 25 February 2021 | archive-url = https://web.archive.org/web/20210225064644/http://www.cedro-uva.org/lib/boekhout.heroine.fr.7.html | url-status = live }}</ref> | ||
| Codeine is also available in conjunction with the anti-nausea medication ] in the form of a syrup. Brand named as Phenergan with Codeine or in generic form as promethazine with |
Codeine is also available in conjunction with the anti-nausea medication ] in the form of a syrup. Brand named as Phenergan with Codeine or in generic form as promethazine with Codeine, it began to be mixed with soft drinks in the 1990s as a recreational drug, called 'syrup', 'lean', or ']'.<ref>{{cite news |newspaper = ] | vauthors = Leinwand D |title = DEA warns of soft drink-cough syrup mix |url = https://www.usatoday.com/news/nation/2006-10-18-lean_x.htm?csp=34 |date = 18 October 2006 |access-date = 23 October 2006 |url-status = live |archive-url = https://web.archive.org/web/20061128201409/http://www.usatoday.com/news/nation/2006-10-18-lean_x.htm?csp=34 |archive-date = 28 November 2006 |df = dmy-all }}</ref> Rapper ], from the group ], died from an overdose of this combination.<ref>{{cite news |url=http://www.chron.com/entertainment/music/article/Pimp-C-s-death-caused-by-overdose-and-sleep-1629962.php |title=Pimp C's death caused by overdose and sleep condition – Houston Chronicle |publisher=Chron.com |date=4 February 2008 |access-date=12 January 2014 |url-status=live |archive-url=https://web.archive.org/web/20131231063510/http://www.chron.com/entertainment/music/article/Pimp-C-s-death-caused-by-overdose-and-sleep-1629962.php |archive-date=31 December 2013 }}</ref> | ||
| Codeine is used in ] to make morphine.<ref>{{Cite book| vauthors = Hogshire J | author-link = Jim Hogshire | title = Pills-A-Go-Go: A Fiendish Investigation into Pill Marketing, Art, History & Consumption | publisher = Feral House |date=June 1999 | location = Los Angeles | pages = 216–223 | isbn = 978-0-922915-53-8}}</ref><ref>{{cite journal |pages=361–70 |doi=10.1134/S1061934808040096 |title=Chromatographic study of expert and biological samples containing desomorphine |year=2011 | vauthors = Savchuk SA, Barsegyan SS, Barsegyan IB, Kolesov GM |journal=Journal of Analytical Chemistry |volume=63 |issue=4|s2cid=54546428 }}</ref> | Codeine is used in ] to make morphine.<ref>{{Cite book| vauthors = Hogshire J | author-link = Jim Hogshire | title = Pills-A-Go-Go: A Fiendish Investigation into Pill Marketing, Art, History & Consumption | publisher = Feral House |date=June 1999 | location = Los Angeles | pages = 216–223 | isbn = 978-0-922915-53-8}}</ref><ref>{{cite journal |pages=361–70 |doi=10.1134/S1061934808040096 |title=Chromatographic study of expert and biological samples containing desomorphine |year=2011 | vauthors = Savchuk SA, Barsegyan SS, Barsegyan IB, Kolesov GM |journal=Journal of Analytical Chemistry |volume=63 |issue=4|s2cid=54546428 }}</ref> | ||
| ===Detection=== | ===Detection=== | ||
| Codeine and its major metabolites may be quantitated in ], ] or ] to monitor therapy, confirm a diagnosis of poisoning or assist in a medico-legal death investigation. ] screening programs generally test ], ], ] or ]. Many commercial opiate screening tests directed at morphine cross-react appreciably with codeine and its metabolites, but chromatographic techniques can easily distinguish codeine from other opiates and opioids. It is important to note that codeine usage results in significant amounts of morphine as an excretion product. Furthermore, ] contains codeine (or acetyl codeine) as an impurity and its use will result in excretion of small amounts of codeine. ] foods represent yet another source of low levels of codeine in one's ]. Blood or plasma codeine concentrations are typically in the 50–300 |
Codeine and its major metabolites may be quantitated in ], ], or ] to monitor therapy, confirm a diagnosis of poisoning, or assist in a medico-legal death investigation. ] screening programs generally test ], ], ] or ]. Many commercial opiate screening tests directed at morphine cross-react appreciably with codeine and its metabolites, but chromatographic techniques can easily distinguish codeine from other opiates and opioids. It is important to note that codeine usage results in significant amounts of morphine as an excretion product. Furthermore, ] contains codeine (or acetyl codeine) as an impurity and its use will result in the excretion of small amounts of codeine. ] foods represent yet another source of low levels of codeine in one's ]. Blood or plasma codeine concentrations are typically in the 50–300 μg/L range in persons taking the drug therapeutically, 700–7,000 μg/L in chronic users, and 1,000–10,000 μg/L in cases of acute fatal over dosage.<ref>{{cite journal | vauthors = Thevis M, Opfermann G, Schänzer W | title = Urinary concentrations of morphine and codeine after consumption of poppy seeds | journal = Journal of Analytical Toxicology | volume = 27 | issue = 1 | pages = 53–56 | year = 2003 | pmid = 12587685 | doi = 10.1093/jat/27.1.53 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Cone EJ, Welch P, Paul BD, Mitchell JM | title = Forensic drug testing for opiates, III. Urinary excretion rates of morphine and codeine following codeine administration | journal = Journal of Analytical Toxicology | volume = 15 | issue = 4 | pages = 161–166 | year = 1991 | pmid = 1943064 | doi = 10.1093/jat/15.4.161 }}</ref><ref>{{cite book |vauthors=Baselt R |title=Disposition of Toxic Drugs and Chemicals in Man |publisher=Biomedical Publications |location=Foster City CA |year=2008 |pages=355–360 |edition=8th }}</ref> | ||
| Codeine is produced in the human body along the ].<ref name="St2012">{{cite journal | vauthors = Stefano GB, Ptáček R, Kuželová H, Kream RM | title = Endogenous morphine: up-to-date review 2011 | journal = Folia Biologica | volume = 58 | issue = 2 | pages = 49–56 | date = 2012 | pmid = 22578954 | url = http://fb.cuni.cz/file/5635/FB2012A0008.pdf | url-status = live | quote = Positive evolutionary pressure has apparently preserved the ability to synthesize chemically authentic morphine, albeit in homeopathic concentrations, throughout animal phyla. | df = dmy-all | archive-url = https://web.archive.org/web/20160824130751/http://fb.cuni.cz/file/5635/FB2012A0008.pdf | archive-date = 24 August 2016 }}</ref> Urinary concentrations of endogenous codeine and morphine have been found to significantly increase in individuals taking ] for the treatment of ].<ref name="St2012" /> | Codeine is produced in the human body along the ].<ref name="St2012">{{cite journal | vauthors = Stefano GB, Ptáček R, Kuželová H, Kream RM | title = Endogenous morphine: up-to-date review 2011 | journal = Folia Biologica | volume = 58 | issue = 2 | pages = 49–56 | date = 2012 | doi = 10.14712/fb2012058020049 | pmid = 22578954 | url = http://fb.cuni.cz/file/5635/FB2012A0008.pdf | url-status = live | quote = Positive evolutionary pressure has apparently preserved the ability to synthesize chemically authentic morphine, albeit in homeopathic concentrations, throughout animal phyla. | df = dmy-all | archive-url = https://web.archive.org/web/20160824130751/http://fb.cuni.cz/file/5635/FB2012A0008.pdf | archive-date = 24 August 2016 }}</ref> Urinary concentrations of endogenous codeine and morphine have been found to significantly increase in individuals taking ] for the treatment of ].<ref name="St2012" /> | ||
| ===Legal status=== | ===Legal status=== | ||
| Around the world, codeine is, contingent on its concentration, a Schedule II and III drug under the ].<ref name="INCB-Yellow">{{cite web | author =International Narcotics Control Board | author-link =International Narcotics Control Board | url =http://www.incb.org/pdf/e/list/46thedition.pdf | title =List of Narcotic Drugs under International Control | access-date =24 May 2006 | url-status =dead | archive-url =https://web.archive.org/web/20120510002957/http://www.incb.org/pdf/e/list/46thedition.pdf | archive-date =10 May 2012 | df =dmy-all }}</ref> In ], ], ], ], the ], the ] and many other countries, codeine is regulated under various ]. In some countries, it is available without a medical prescription in combination preparations from licensed pharmacists in doses up to 20 mg, or 30 mg when sold combined with 500 mg paracetamol. | Around the world, codeine is, contingent on its concentration, a Schedule II and III drug under the ].<ref name="INCB-Yellow">{{cite web | author =International Narcotics Control Board | author-link =International Narcotics Control Board | url =http://www.incb.org/pdf/e/list/46thedition.pdf | title =List of Narcotic Drugs under International Control | access-date =24 May 2006 | url-status =dead | archive-url =https://web.archive.org/web/20120510002957/http://www.incb.org/pdf/e/list/46thedition.pdf | archive-date =10 May 2012 | df =dmy-all }}</ref> In ], ], ], ], the ], the ] and many other countries, codeine is regulated under various ]. In some countries, it is available without a medical prescription in combination preparations from licensed pharmacists in doses up to 20 mg, or 30 mg when sold combined with 500 mg paracetamol. | ||
| As of 2015, of the European Union member states, 11 countries (Bulgaria, Cyprus, Denmark, Estonia, Ireland, Latvia, Lithuania, Malta, Poland, Romania, and Slovenia) allow the sale of OTC codeine solid dosage forms.<ref name="academia.edu">{{cite journal | vauthors = Foley M, Harris R, Rich E, Rapca A, Bergin M, Norman I, Van Hout MC | title = The availability of over-the-counter codeine medicines across the European Union | journal = Public Health | volume = 129 | issue = 11 | pages = 1465–1470 | date = November 2015 | pmid = 26215740 | doi = 10.1016/j.puhe.2015.06.014 | url = https://www.academia.edu/19659390 | access-date = 8 July 2020 | url-status = live | archive-url = https://web.archive.org/web/20200709021531/https://www.academia.edu/19659390/The_availability_of_over-the-counter_codeine_medicines_across_the_European_Union | archive-date = 9 July 2020 }}</ref> | |||
| ====Australia==== | ====Australia==== | ||
| Line 306: | Line 300: | ||
| ====Estonia==== | ====Estonia==== | ||
| Until 2023, in Estonia codeine was sold over the counter in dosages up to 8 mg (with paracetamol, brand name Co-Codamol).<ref>{{Cite web | |
Until 2023, in Estonia codeine was sold over the counter in dosages up to 8 mg (with paracetamol, brand name Co-Codamol).<ref>{{Cite web | vauthors = Koppel K |date=2022-11-23 |title=Pharmacists welcome ban on OTC sale of painkillers containing codeine |url=https://news.err.ee/1608797869/pharmacists-welcome-ban-on-otc-sale-of-painkillers-containing-codeine |access-date=2023-11-08 |website=ERR |language=en}}</ref> | ||
| ====France==== | ====France==== | ||
| Line 315: | Line 309: | ||
| ====Hong Kong==== | ====Hong Kong==== | ||
| In Hong Kong |
In Hong Kong, codeine is regulated under the Laws of the Hong Kong, Dangerous Drugs Ordinance, Chapter 134, Schedule 1. It can be used legally only by health professionals and for university research purposes. The substance can be given by pharmacists under a prescription. Anyone who supplies the substance without a prescription can be fined $10,000 (]). The maximum penalty for trafficking or manufacturing the substance is a $5,000,000 (HKD) fine and life imprisonment. Possession of the substance for consumption without license from the Department of Health is illegal with a $1,000,000 (HKD) fine and/or 7 years of jail time. | ||
| However, codeine is available without prescription from licensed pharmacists in doses up to 0.1%<ref name=LawsOfHongKongChapter134>Laws of Hong Kong, Dangerous Drugs Ordinance, Chapter 134 {{cite web |url=http://www.legislation.gov.hk/eng/home.htm |title=Hong Kong e-Legislation |access-date=16 October 2016 |url-status=dead |archive-url=https://web.archive.org/web/20121016064430/http://www.legislation.gov.hk/eng/home.htm |archive-date=16 October 2012 }}</ref>{{rp|Schedule 1, Part IV, paragraph 23}} (i.e. 5 mg/5ml)<ref name=LawsOfHongKongChapter134 />{{rp|Section 3, (1) (a)}} | However, codeine is available without prescription from licensed pharmacists in doses up to 0.1%<ref name=LawsOfHongKongChapter134>Laws of Hong Kong, Dangerous Drugs Ordinance, Chapter 134 {{cite web |url=http://www.legislation.gov.hk/eng/home.htm |title=Hong Kong e-Legislation |access-date=16 October 2016 |url-status=dead |archive-url=https://web.archive.org/web/20121016064430/http://www.legislation.gov.hk/eng/home.htm |archive-date=16 October 2012 }}</ref>{{rp|Schedule 1, Part IV, paragraph 23}} (i.e. 5 mg/5ml)<ref name=LawsOfHongKongChapter134 />{{rp|Section 3, (1) (a)}} | ||
| Line 338: | Line 332: | ||
| ====South Africa==== | ====South Africa==== | ||
| Codeine is available over the counter in South Africa. Certain pharmacies require people to write down their name and address to ensure they are not buying too much over a short period although many do not require this at all. According to Lochan Naidoo, the former president of the National Narcotics Control Board, making the drugs more difficult to obtain could lead to even worse problems where people in withdrawal would turn to illicit drugs to get their fix.<ref>{{cite web |url=http://www.iol.co.za/news/south-africa/kwazulu-natal/sas-silent-codeine-addiction-uncovered-2071990 |title=SA's silent codeine addiction uncovered |
Codeine is available over the counter in South Africa. Certain pharmacies require people to write down their name and address to ensure they are not buying too much over a short period although many do not require this at all. According to Lochan Naidoo, the former president of the National Narcotics Control Board, making the drugs more difficult to obtain could lead to even worse problems where people in withdrawal would turn to illicit drugs to get their fix.<ref>{{cite web |url=http://www.iol.co.za/news/south-africa/kwazulu-natal/sas-silent-codeine-addiction-uncovered-2071990 |title=SA's silent codeine addiction uncovered {{pipe}} IOL News |access-date=16 October 2016 |url-status=live |archive-url=https://web.archive.org/web/20161017035926/http://www.iol.co.za/news/south-africa/kwazulu-natal/sas-silent-codeine-addiction-uncovered-2071990 |archive-date=17 October 2016 }}</ref> Although codeine is freely available, South Africa has a fairly low annual prevalence rate of opiate use at 0.3% compared to the United States at 0.57% where all opiates are strictly regulated. | ||
| ====United Arab Emirates==== | ====United Arab Emirates==== | ||
| Line 351: | Line 345: | ||
| ====United States==== | ====United States==== | ||
| In the United States, codeine is regulated by the ]. Federal law dictates that codeine be a Schedule II controlled substance when used in products for pain |
In the United States, codeine is regulated by the ]. Federal law dictates that codeine be a Schedule II controlled substance when used in products for pain relief that contain codeine alone or more than 80 mg per dosage unit. Codeine without aspirin or acetaminophen (Tylenol) is very rarely available or prescribed to discourage abuse. Tablets of codeine in combination with aspirin or acetaminophen (]) and intended for pain relief are listed as Schedule III. | ||
| Cough syrups are classed as Schedule III, IV or V, depending on formulation. For example, the acetaminophen/codeine antitussive liquid is a Schedule IV controlled substance.<ref name="Valeant Pharmaceuticals Prescribing Information for Capital with Codeine Oral Suspension">{{cite web | author =Valeant Pharmaceuticals | author-link =Valeant Pharmaceuticals | url =http://www.valeant.com/fileRepository/products/PI/Capital_with_Codeine_Suspension_120mg_PI_Aug04.pdf | title =Prescribing Information for Capital with Codeine (paracetamol with codeine) showing Schedule V designation | access-date =25 February 2011 | url-status =dead | archive-url =https://web.archive.org/web/20110717183303/http://www.valeant.com/fileRepository/products/PI/Capital_with_Codeine_Suspension_120mg_PI_Aug04.pdf | archive-date =17 July 2011 | df =dmy-all }}</ref> | Cough syrups are classed as Schedule III, IV, or V, depending on formulation. For example, the acetaminophen/codeine antitussive liquid is a Schedule IV controlled substance.<ref name="Valeant Pharmaceuticals Prescribing Information for Capital with Codeine Oral Suspension">{{cite web | author =Valeant Pharmaceuticals | author-link =Valeant Pharmaceuticals | url =http://www.valeant.com/fileRepository/products/PI/Capital_with_Codeine_Suspension_120mg_PI_Aug04.pdf | title =Prescribing Information for Capital with Codeine (paracetamol with codeine) showing Schedule V designation | access-date =25 February 2011 | url-status =dead | archive-url =https://web.archive.org/web/20110717183303/http://www.valeant.com/fileRepository/products/PI/Capital_with_Codeine_Suspension_120mg_PI_Aug04.pdf | archive-date =17 July 2011 | df =dmy-all }}</ref> | ||
| Some states have chosen to reclassify codeine preparations at a more restrictive schedule |
Some states have chosen to reclassify codeine preparations at a more restrictive schedule to lower the instances of its abuse. Minnesota, for instance, has chosen to reclassify Schedule V some codeine preparations (e.g. ]) as a Schedule III controlled substance.<ref name="Minnesota; Schedules of controlled substances">{{cite web|title=152.02 Schedules Of Controlled Substances|url=https://www.revisor.mn.gov/statutes/?id=152.02|publisher=State of Minnesota|access-date=30 May 2013|url-status=live|archive-url=https://web.archive.org/web/20130730103623/https://www.revisor.mn.gov/statutes/?id=152.02|archive-date=30 July 2013}}</ref> | ||
| =====Schedule V controlled substances===== | =====Schedule V controlled substances===== | ||
| Line 361: | Line 355: | ||
| Substances in this schedule have a low potential for abuse relative to substances listed in Schedule IV and consist primarily of preparations containing limited quantities of certain narcotics. | Substances in this schedule have a low potential for abuse relative to substances listed in Schedule IV and consist primarily of preparations containing limited quantities of certain narcotics. | ||
| Examples of Schedule V substances include |
Examples of Schedule V substances include cough preparations containing not more than 200 milligrams of codeine per 100 milliliters or per 100 grams (Robitussin AC, Phenergan with Codeine).<ref> {{Webarchive|url=https://web.archive.org/web/20201121231025/https://www.deadiversion.usdoj.gov/schedules/ |date=21 November 2020 }} U.S. DEPARTMENT OF JUSTICE, DRUG ENFORCEMENT ADMINISTRATION: Diversion Control Division.</ref> | ||
| == References == | == References == | ||
| Line 374: | Line 368: | ||
| == External links == | == External links == | ||
| {{Commons category|Codeine}} | {{Commons category|Codeine}} | ||
| * {{cite web| url = https://druginfo.nlm.nih.gov/drugportal/rn/76-57-3 | publisher = U.S. National Library of Medicine| work = Drug Information Portal | title = Codeine }} | |||
| {{Components of Opium}} | {{Components of Opium}} | ||
Latest revision as of 00:56, 25 December 2024
Opiate and prodrug of morphine used to treat pain For other uses, see Codeine (disambiguation).Pharmaceutical compound
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˈkoʊdiːn/ |
| Other names | 3-Methylmorphine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682065 |
| License data | |
| Pregnancy category |
|
| Dependence liability | High |
| Addiction liability | High |
| Routes of administration | By mouth, rectal, subcutaneous, intramuscular |
| Drug class | |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | c. 60% (by mouth) |
| Metabolism | Liver: CYP2D6 (to morphine), CYP3A4 (to norcodeine), UGT2B7 (to 3- and 6-glucuronides of codeine, norcodeine, and morphine) |
| Metabolites |
|
| Onset of action | 15–30 minutes |
| Elimination half-life | 2.5–3 hours |
| Duration of action | 4–6 hours |
| Identifiers | |
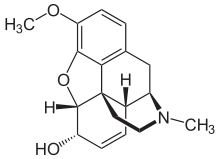
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.882 |
| Chemical and physical data | |
| Formula | C18H21NO3 |
| Molar mass | 299.370 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Codeine is an opiate and prodrug of morphine mainly used to treat pain, coughing, and diarrhea. It is also commonly used as a recreational drug. It is found naturally in the sap of the opium poppy, Papaver somniferum. It is typically used to treat mild to moderate degrees of pain. Greater benefit may occur when combined with paracetamol (acetaminophen) or a nonsteroidal anti-inflammatory drug (NSAID) such as aspirin or ibuprofen. Evidence does not support its use for acute cough suppression in children. In Europe, it is not recommended as a cough medicine for those under 12 years of age. It is generally taken by mouth. It typically starts working after half an hour, with maximum effect at two hours. Its effects last for about four to six hours. Codeine exhibits abuse potential similar to other opioid medications, including a risk of addiction and overdose.
Common side effects include vomiting, constipation, itchiness, lightheadedness, and drowsiness. Serious side effects may include breathing difficulties and addiction. Whether its use in pregnancy is safe is unclear. Care should be used during breastfeeding, as it may result in opiate toxicity in the baby. Its use as of 2016 is not recommended in children. Codeine works following being broken down by the liver into morphine; how quickly this occurs depends on a person's genetics.
Codeine was discovered in 1832 by Pierre Jean Robiquet. In 2013, about 361,000 kg (795,000 lb) of codeine were produced while 249,000 kg (549,000 lb) were used, which made it the most commonly taken opiate. It is on the World Health Organization's List of Essential Medicines. Codeine occurs naturally and makes up about 2% of opium.
Medical uses
Pain
Codeine is used to treat mild to moderate pain. It is commonly used to treat post-surgical dental pain.
Weak evidence indicates that it is useful in cancer pain, but it may have increased adverse effects, especially constipation, compared to other opioids. The American Academy of Pediatrics does not recommend its use in children due to side effects. The Food and Drug Administration (FDA) lists age under 12 years old as a contraindication to use.
Cough
Codeine is used to relieve coughing. Evidence does not support its use for acute cough suppression in children. In Europe, it is not recommended as a cough medicine for those under 12 years of age. Some tentative evidence shows it can reduce a chronic cough in adults.
Diarrhea
It is used to treat diarrhea and diarrhea-predominant irritable bowel syndrome, although loperamide (which is available without a prescription for milder diarrhea), diphenoxylate, paregoric, or even laudanum are more frequently used to treat severe diarrhea.
Formulations
| This section does not cite any sources. Please help improve this section by adding citations to reliable sources. Unsourced material may be challenged and removed. (January 2023) (Learn how and when to remove this message) |
Codeine is marketed as both a single-ingredient drug and in combination preparations with paracetamol (as co-codamol: e.g., brands Paracod, Panadeine, and the Tylenol-with-codeine series, including Tylenol 3 and 1, 2, and 4); with aspirin (as co-codaprin); or with ibuprofen (as Nurofen Plus). These combinations provide greater pain relief than either agent alone (drug synergy).
Codeine is also commonly marketed in products containing codeine with other pain killers or muscle relaxers, as well as codeine mixed with phenacetin (Emprazil with codeine No. 1, 2, 3, 4, and 5), naproxen, indomethacin, diclofenac, and others, as well as more complex mixtures, including such mixtures as aspirin + paracetamol + codeine ± caffeine ± antihistamines and other agents, such as those mentioned above.
Codeine-only products can be obtained with a prescription as a time-release tablet. Codeine is also marketed in cough syrups with zero to a half-dozen other active ingredients, and a linctus (e.g., Paveral) for all of the uses for which codeine is indicated.
Injectable codeine is available for subcutaneous or intramuscular injection only; intravenous injection is contraindicated, as this can result in nonimmune mast-cell degranulation and resulting anaphylactoid reaction. Codeine suppositories are also marketed in some countries.
Side effects
Common adverse effects associated with the use of codeine include drowsiness and constipation. Less common are itching, nausea, vomiting, dry mouth, miosis, orthostatic hypotension, urinary retention, euphoria, and dysphoria. Rare adverse effects include anaphylaxis, seizure, acute pancreatitis, and respiratory depression. As with all opiates, long-term effects can vary, but can include diminished libido, apathy, and memory loss. Some people may have allergic reactions to codeine, such as the swelling of the skin and rashes.
Tolerance to many of the effects of codeine, including its therapeutic effects, develops with prolonged use. This occurs at different rates for different effects, with tolerance to the constipation-inducing effects developing particularly slowly for instance.
As with other opioids, a potentially serious adverse drug reaction is respiratory depression. This depression is dose-related and is a mechanism for the potentially fatal consequences of overdose. As codeine is metabolized to morphine, morphine can be passed through breast milk in potentially lethal amounts, fatally depressing the respiration of a breastfed baby. In August 2012, the United States Food and Drug Administration issued a warning about deaths in pediatric patients less than 6 years old after ingesting "normal" doses of paracetamol with codeine after tonsillectomy; this warning was upgraded to a black box warning in February 2013.
Some patients are very effective converters of codeine to its active form, morphine, resulting in lethal blood levels. The FDA is presently recommending very cautious use of codeine in young tonsillectomy patients; the drug should be used in the lowest amount that can control the pain, "as needed" and not "around the clock", and immediate medical attention is needed if the user responds negatively.
Withdrawal and dependence
As with other opiates, chronic use of codeine can cause physical dependence which can lead to severe withdrawal symptoms if a person suddenly stops the medication. Withdrawal symptoms include drug craving, runny nose, yawning, sweating, insomnia, weakness, stomach cramps, nausea, vomiting, diarrhea, muscle spasms, chills, irritability, and pain. These side effects also occur in acetaminophen/aspirin combinations, though to a lesser extent. To minimize withdrawal symptoms, long-term users should gradually reduce their codeine medication under the supervision of a healthcare professional.
Also, no evidence indicates that CYP2D6 inhibition is useful in treating codeine dependence, though the metabolism of codeine to morphine (and hence further metabolism to glucuronide morphine conjugates) does have an effect on the abuse potential of codeine. However, CYP2D6 has been implicated in the toxicity and death of neonates when codeine is administered to lactating mothers, particularly those with increased enzyme activity ("ultra-rapid" metabolizers).
In 2019 Ireland was said to be on the verge of a codeine addiction epidemic, according to a paper in the Irish Medical Journal. Under Irish law, codeine can be bought over the counter under the supervision of a pharmacist, but there is no mechanism to detect patients travelling to different pharmacies to purchase codeine.
Pharmacology
Pharmacodynamics
| Compound | Affinities (KiTooltip Inhibitor constant) | Ratio | Ref | ||
|---|---|---|---|---|---|
| MORTooltip μ-Opioid receptor | DORTooltip δ-Opioid receptor | KORTooltip κ-Opioid receptor | MOR:DOR:KOR | ||
| Codeine | 79 nM | >1,000 nM | >1,000 nM | ND | |
| Morphine | 1.8 nM | 90 nM | 317 nM | 1:50:176 | |
| Compound | Route | Dose |
|---|---|---|
| Codeine | PO | 200 mg |
| Hydrocodone | PO | 20–30 mg |
| Hydromorphone | PO | 7.5 mg |
| Hydromorphone | IV | 1.5 mg |
| Morphine | PO | 30 mg |
| Morphine | IV | 10 mg |
| Oxycodone | PO | 20 mg |
| Oxycodone | IV | 10 mg |
| Oxymorphone | PO | 10 mg |
| Oxymorphone | IV | 1 mg |
Codeine is a nonsynthetic opioid. It is a selective agonist of the μ-opioid receptor (MOR). Codeine itself has relatively weak affinity for the MOR. Instead of acting directly on the MOR, codeine functions as a prodrug of its major active metabolites morphine and codeine-6-glucuronide, which are far more potent MOR agonists in comparison.
Codeine has been found as an endogenous compound, along with morphine, in the brains of nonhuman primates with depolarized neurons, indicating that codeine may function as a neurotransmitter or neuromodulator in the central nervous system. Like morphine, codeine causes TLR4 signaling which causes allodynia and hyperalgesia. It does not need to be converted to morphine to increase pain sensitivity.
Mechanism of action
Codeine is an opiate and an agonist of the mu opioid receptor (MOR). It acts on the central nervous system to have an analgesic effect. It is metabolised in the liver to produce morphine which is ten times more potent against the mu receptor. Opioid receptors are G protein-coupled receptors that positively and negatively regulate synaptic transmission through downstream signalling. Binding of codeine or morphine to the mu-opioid receptor results in hyperpolarization of the neuron leading to the inhibition of the release of nociceptive neurotransmitters, causing an analgesic effect and increased pain tolerance due to reduced neuronal excitability.
Pharmacokinetics
The conversion of codeine to morphine occurs in the liver and is catalyzed by the cytochrome P450 enzyme CYP2D6. CYP3A4 produces norcodeine, and UGT2B7 conjugates codeine, norcodeine, and morphine to the corresponding 3- and 6-glucuronides. Srinivasan, Wielbo, and Tebbett speculate that codeine-6-glucuronide is responsible for a large percentage of the analgesia of codeine, and thus these patients should experience some analgesia. Many of the adverse effects will still be experienced in poor metabolizers. Conversely, between 0.5% and 2% of the population are "extensive metabolizers"; multiple copies of the gene for 2D6 produce high levels of CYP2D6 and will metabolize drugs through that pathway more quickly than others.
Some medications are CYP2D6 inhibitors and reduce or even completely block the conversion of codeine to morphine. The best-known of these are two of the selective serotonin reuptake inhibitors, paroxetine (Paxil) and fluoxetine (Prozac) as well as the antihistamine diphenhydramine (Benadryl) and the antidepressant bupropion (Wellbutrin, also known as Zyban). Other drugs, such as rifampicin and dexamethasone, induce CYP450 isozymes and thus increase the conversion rate.
CYP2D6 converts codeine into morphine, which then undergoes glucuronidation. Life-threatening intoxication, including respiratory depression requiring intubation, can develop over a matter of days in patients who have multiple functional alleles of CYP2D6, resulting in ultrarapid metabolism of opioids such as codeine into morphine.
Studies on codeine's analgesic effect are consistent with the idea that metabolism by CYP2D6 to morphine is important, but some studies show no major differences between those who are poor metabolizers and extensive metabolizers. Evidence supporting the hypothesis that ultrarapid metabolizers may get greater analgesia from codeine due to increased morphine formation is limited to case reports.
Due to the increased metabolism of codeine to morphine, ultrarapid metabolizers (those possessing more than two functional copies of the CYP2D6 allele) are at increased risk of adverse drug effects related to morphine toxicity. Guidelines released by the Clinical Pharmacogenomics Implementation Consortium (CPIC) advise against administering codeine to ultrarapid metabolizers, where this genetic information is available. The CPIC also suggests that codeine use be avoided in poor metabolizers, due to its lack of efficacy in this group.
Codeine and its salts are readily absorbed from the gastrointestinal tract, and ingestion of codeine phosphate produces peak plasma concentrations in about one hour. Plasma half life is between 3 and 4 hours, and oral/intramuscular analgesic potency ratio is approximately equal to 1:1.5. The most common conversion ratio, given on equianalgesia charts used in the United States, Canada, the UK, Republic of Ireland, the European Union, Russia and elsewhere as 130 mg IM equals 200 mg PO—both of which are equivalent to 10 mg of morphine sulphate IV and 60 mg of morphine sulphate PO. The salt:freebase ratio of the salts of both drugs in use are roughly equivalent, and do not generally make a clinical difference.
Codeine is metabolised by O- and N-demethylation in the liver to morphine and norcodeine. Hydrocodone is also a metabolite of codeine in humans. Codeine and its metabolites are mostly removed from the body by the kidneys, primarily as conjugates with glucuronic acid.
The active metabolites of codeine, notably morphine, exert their effects by binding to and activating the μ-opioid receptor. In people that can extensively metabolize the codeine, a 30 mg dose could yield up to 4 mg of morphine.
Chemistry
While codeine can be directly extracted from opium, its source, most codeine is synthesized from the much more abundant morphine through the process of O-methylation, through a process first completed in the late 20th century by Robert C. Corcoran and Junning Ma.
Relation to other opioids
Codeine has been used in the past as the starting material and prototype of a large class of mainly mild to moderately strong opioids, such as hydrocodone (1920 in Germany), oxycodone (1916 in Germany), dihydrocodeine (1908 in Germany), and its derivatives such as nicocodeine (1956 in Austria). However, these opioids are no longer synthesized from codeine and are usually synthesized from other opium alkaloids, specifically thebaine. Other series of codeine derivatives include isocodeine and its derivatives, which were developed in Germany starting around 1920. In general, the various classes of morphine derivatives such as ketones, semisynthetics like dihydromorphine, halogeno-morphides, esters, ethers, and others have codeine, dihydrocodeine, and isocodeine analogues. The codeine ester acetylcodeine is a common active impurity in street heroin as some codeine tends to dissolve with the morphine when it is extracted from opium in underground heroin and morphine base labs.
As an analgesic, codeine compares weakly to other opiates. Related to codeine in other ways are codoxime, thebacon, codeine-N-oxide (genocodeine), related to the nitrogen morphine derivatives as is codeine methobromide, and heterocodeine, which is a drug six times stronger than morphine and 72 times stronger than codeine due to a small re-arrangement of the molecule, namely moving the methyl group from the 3 to the 6 position on the morphine carbon skeleton.
Drugs bearing resemblance to codeine in effects due to close structural relationship are variations on the methyl groups at the 3 position including ethylmorphine, also known as codethyline (Dionine), and benzylmorphine (Peronine). While having no narcotic effects of its own, the important opioid precursor thebaine differs from codeine only slightly in structure. Pseudocodeine and some other similar alkaloids not currently used in medicine are found in trace amounts in opium as well.
History
Codeine, or 3-methylmorphine, is an alkaloid found in the opium poppy, Papaver somniferum var. album, a plant in the family Papaveraceae. Opium poppy has been cultivated and utilized throughout human history for a variety of medicinal (analgesic, anti-tussive and anti-diarrheal) and hypnotic properties linked to the diversity of its active components, which include morphine, codeine and papaverine.
Codeine is found in concentrations of 1% to 3% in opium prepared by the latex method from unripe pods of Papaver somniferum. The name codeine is derived from the Ancient Greek κώδεια (kṓdeia, "poppy head"). The relative proportion of codeine to morphine, the most common opium alkaloid at 4% to 23%, tends to be somewhat higher in the poppy straw method of preparing opium alkaloids.
Until the beginning of the 19th century, raw opium was used in diverse preparations known as laudanum (see Thomas de Quincey's Confessions of an English Opium-Eater, 1821) and paregoric elixirs, several which were popular in England since the beginning of the 18th century; the original preparation seems to have been elaborated in Leiden, the Netherlands around 1715 by a chemist Jakob Le Mort; in 1721 the London Pharmacopoeia mentions an Elixir Asthmaticum, replaced by the term Elixir Paregoricum ("pain soother") in 1746.
The progressive isolation of opium's several active components opened the path to improved selectivity and safety of the opiates-based pharmacopeia.
Morphine had already been isolated in Germany by Friedrich Sertürner in 1804. Codeine was first isolated in 1832 in France by Pierre Robiquet, already famous for the discovery of alizarin, the most widespread red dye, while working on refined morphine extraction processes. Robiquet is also credited with discovering caffeine independently of Pelletier, Caventou, and Runge. Thomas Anderson determined the correct composition in 1853 but a chemical structure was proposed only in 1925 by J. M. Gulland and Robert Robinson. The first crystal structure would have to wait until 1954.
Codeine and morphine, as well as opium, were used in an attempt to treat diabetes in the 1880s and thereafter, as recently as the 1950s.
Numerous codeine salts have been prepared since the drug was discovered. The most commonly used are the hydrochloride (freebase conversion ratio 0.805, i.e. 10 mg of the hydrochloride salt is equivalent in effect to 8.05 mg of the freebase form), phosphate (0.736), sulphate (0.859), and citrate (0.842). Others include a salicylate NSAID, codeine salicylate (0.686), a bromide (codeine methylbromide, 0.759), and at least five codeine-based barbiturates, the phenylethylbarbiturate (0.56), cyclohexenylethylbarbiturate (0.559), cyclopentenylallylbarbiturate (0.561), diallylbarbiturate (0.561), and diethylbarbiturate (0.619). The latter was introduced as Codeonal in 1912, indicated for pain with nervousness. Codeine methylbromide is also considered a separate drug for various purposes.
Society and culture
Codeine is the most widely used opiate in the world, and is one of the most commonly used drugs overall according to numerous reports by organizations including the World Health Organization and its League of Nations predecessor agency.
Names
It is often sold as a salt in the form of either codeine sulfate or codeine phosphate in the United States, United Kingdom, and Australia. Codeine hydrochloride is more common worldwide and the citrate, hydroiodide, hydrobromide, tartrate, and other salts are also seen. The chemical name for codeine is morphinan-6-ol, 7,8-didehydro-4,5-epoxy-3-methoxy-17-methyl-, (5α,6α)-
Recreational use

A heroin (diamorphine) or other opiate/opioid addict may use codeine to ward off the effects of withdrawal during periods where their preferred drug is unavailable or unaffordable.
Codeine is also available in conjunction with the anti-nausea medication promethazine in the form of a syrup. Brand named as Phenergan with Codeine or in generic form as promethazine with Codeine, it began to be mixed with soft drinks in the 1990s as a recreational drug, called 'syrup', 'lean', or 'purple drank'. Rapper Pimp C, from the group UGK, died from an overdose of this combination.
Codeine is used in illegal drug laboratories to make morphine.
Detection
Codeine and its major metabolites may be quantitated in blood, plasma, or urine to monitor therapy, confirm a diagnosis of poisoning, or assist in a medico-legal death investigation. Drug abuse screening programs generally test urine, hair, sweat or saliva. Many commercial opiate screening tests directed at morphine cross-react appreciably with codeine and its metabolites, but chromatographic techniques can easily distinguish codeine from other opiates and opioids. It is important to note that codeine usage results in significant amounts of morphine as an excretion product. Furthermore, heroin contains codeine (or acetyl codeine) as an impurity and its use will result in the excretion of small amounts of codeine. Poppy seed foods represent yet another source of low levels of codeine in one's biofluids. Blood or plasma codeine concentrations are typically in the 50–300 μg/L range in persons taking the drug therapeutically, 700–7,000 μg/L in chronic users, and 1,000–10,000 μg/L in cases of acute fatal over dosage.
Codeine is produced in the human body along the same biosynthetic pathway as morphine. Urinary concentrations of endogenous codeine and morphine have been found to significantly increase in individuals taking L-DOPA for the treatment of Parkinson's disease.
Legal status
Around the world, codeine is, contingent on its concentration, a Schedule II and III drug under the Single Convention on Narcotic Drugs. In Australia, Canada, New Zealand, Sweden, the United Kingdom, the United States and many other countries, codeine is regulated under various narcotic control laws. In some countries, it is available without a medical prescription in combination preparations from licensed pharmacists in doses up to 20 mg, or 30 mg when sold combined with 500 mg paracetamol.
As of 2015, of the European Union member states, 11 countries (Bulgaria, Cyprus, Denmark, Estonia, Ireland, Latvia, Lithuania, Malta, Poland, Romania, and Slovenia) allow the sale of OTC codeine solid dosage forms.
Australia
In Australia, since 1 February 2018, preparations containing codeine are not available without a prescription.
Preparations containing pure codeine (e.g., codeine phosphate tablets or codeine phosphate linctus) are available on prescription and are considered S8 (Schedule 8, or "Controlled Drug Possession without authority illegal"). Schedule 8 preparations are subject to the strictest regulation of all medications available to consumers.
Prior to 1 February 2018, Codeine was available over-the-counter (OTC).
Canada
In Canada, codeine is regulated under the Narcotic Control Regulations (NCR), which falls under the Controlled Drugs and Substances Act (CDSA). Regulations state the pharmacists may, without a prescription, sell low-dose codeine products (containing up to 8 mg of codeine per tablet or up to 20 mg per 30 ml in liquid preparation) if the preparation contains at least two additional medicinal ingredients other than a narcotic (S.36.1 NCR).
In Canada tablets containing 8 mg of codeine combined with 15 mg of caffeine and 300 mg of acetaminophen are sold as T1s (Tylenol Number 1) without a prescription. A similar tablet called "A.C. & C." (which stands for Acetylsalicylic acid with Caffeine and Codeine) containing 325–375 mg of acetylsalicylic acid (Aspirin) instead of acetaminophen is also available without a prescription. Codeine combined with an antihistamine, and often caffeine, is sold under various trade names and is available without a prescription. These products are kept behind the counter and must be dispensed by a pharmacist who may limit quantities.
Names of many codeine and dihydrocodeine products in Canada tend to follow the narcotic content number system (Tylenol With Codeine No. 1, 2, 3, 4 &c) mentioned below in the section on the United States; it came to be in its current form with the Pure Food & Drug Act of 1906.
Controlled Drugs and Substances Act (S.C. 1996, c. 19) effective 28 July 2020. Codeine is now classified under Schedule 1, giving it a higher priority in the treatments of offenders of the law.
Codeine became a prescription-only medication in the province of Manitoba on 1 February 2016. The number of low-dose codeine tablets sold in Manitoba decreased by 94 percent from 52.5 million tablets sold in the year prior to the policy change to 3.3 million in the year after. A pharmacist may issue a prescription, and all purchases are logged to a central database to prevent overprescribing. Saskatchewan's pharmacy college is considering enacting a similar ban to Manitoba's.
On 9 May 2019, the Canadian Pharmacists Association wrote to Health Canada proposing regulations amending the NCR, the BOTSR, and the FDR - Part G, which included requiring that all products containing codeine be available by prescription only.
New safety measures were issued by Health Canada on 28 July 2016; "codeine should no longer be used (contraindicated) in patients under 18 years of age to treat pain after surgery to remove tonsils or adenoids, as these patients are more susceptible to the risk of serious breathing problems. Codeine (prescription and non-prescription) is already not recommended for children under the age of 12, for any use."
Denmark
In Denmark codeine is sold over the counter in dosages up to 9.6 mg (with aspirin, brand name Kodimagnyl); anything stronger requires a prescription.
Estonia
Until 2023, in Estonia codeine was sold over the counter in dosages up to 8 mg (with paracetamol, brand name Co-Codamol).
France
In France, most preparations containing codeine only began requiring a doctor's prescription in 2017. Products containing codeine include Néocodion (codeine and camphor), Tussipax (ethylmorphine and codeine), Paderyl (codeine alone), Codoliprane (codeine with paracetamol), Prontalgine and Migralgine (codeine, paracetamol and caffeine). The 2017 law change made a prescription mandatory for all codeine products, along with those containing ethylmorphine and dextromethorphan.
Greece
Codeine is classed as an illegal drug in Greece, and individuals possessing it could conceivably be arrested, even if they were legitimately prescribed it in another country. It is sold only with a doctor's prescription (Lonarid-N, Lonalgal).
Hong Kong
In Hong Kong, codeine is regulated under the Laws of the Hong Kong, Dangerous Drugs Ordinance, Chapter 134, Schedule 1. It can be used legally only by health professionals and for university research purposes. The substance can be given by pharmacists under a prescription. Anyone who supplies the substance without a prescription can be fined $10,000 (HKD). The maximum penalty for trafficking or manufacturing the substance is a $5,000,000 (HKD) fine and life imprisonment. Possession of the substance for consumption without license from the Department of Health is illegal with a $1,000,000 (HKD) fine and/or 7 years of jail time.
However, codeine is available without prescription from licensed pharmacists in doses up to 0.1% (i.e. 5 mg/5ml)
India
Codeine preparations require a prescription in India. A preparation of paracetamol and codeine is available in India. Codeine is also present in various cough syrups as codeine phosphate including chlorpheniramine maleate. Pure codeine is also available as codeine sulphate tablets. Codeine containing cough medicine has been banned in India with effect from 14 March 2016. The Ministry of Health and Family Welfare has found no proof of its efficacy against cough control.
Ireland
In Ireland, new regulations came into effect on 1 August 2010 concerning codeine, due to worries about the overuse of the drug. Codeine remains a semi non-prescriptive, over-the-counter drug up to a limit of 12.8 mg per pill, but codeine products must be out of the view of the public to facilitate the legislative requirement that these products "are not accessible to the public for self-selection". In practice, this means customers must ask pharmacists for the product containing codeine in name, and the pharmacist makes a judgement whether it is suitable for the patient to be using codeine, and that patients are fully advised of the correct use of these products. Products containing more than 12.8 mg codeine are available on prescription only.
Italy
Codeine tablets or preparations require a prescription in Italy. Preparations of paracetamol and codeine are available in Italy as Co-Efferalgan and Tachidol.
Japan
Codeine is available over the counter at pharmacies, allowing up to 50 mg of codeine phosphate per day for adults.
Latvia
In Latvia codeine is sold over the counter in dosages up to 8 mg (with paracetamol, brand name Co-Codamol).
Nigeria
Nigeria in 2018 plans to ban the manufacture and import of cough syrup that include codeine as an ingredient. This is due to concerns regarding its use to get intoxicated.
South Africa
Codeine is available over the counter in South Africa. Certain pharmacies require people to write down their name and address to ensure they are not buying too much over a short period although many do not require this at all. According to Lochan Naidoo, the former president of the National Narcotics Control Board, making the drugs more difficult to obtain could lead to even worse problems where people in withdrawal would turn to illicit drugs to get their fix. Although codeine is freely available, South Africa has a fairly low annual prevalence rate of opiate use at 0.3% compared to the United States at 0.57% where all opiates are strictly regulated.
United Arab Emirates
The UAE takes an exceptionally strict line on medicines, with many common drugs, notably anything containing codeine being banned unless one has a notarized and authenticated doctor's prescription. Visitors breaking the rules, even inadvertently, have been deported or imprisoned. The US Embassy to the UAE maintains an unofficial list of what may not be imported.
United Kingdom
In the United Kingdom, the sale and possession of codeine are restricted separately under law.
Neat codeine and higher-strength codeine formulations are generally prescription-only medicines (POM) meaning that the sale of such products is restricted under the Medicines Act 1968. Lower-strength products containing combinations of up to 12.8 mg of codeine per dosage unit, combined with paracetamol, ibuprofen or aspirin are available over the counter at pharmacies. Codeine linctus of 15 mg per 5 ml is also available at some pharmacies, although a purchaser would have to request it specifically from the pharmacist.
Under the Misuse of Drugs Act 1971 codeine is a Class B controlled substance or a Class A drug when prepared for injection. The possession of controlled substances without a prescription is a criminal offence. However, certain preparations of codeine are exempt from this restriction under Schedule 5 of the Misuse of Drugs Regulations 2001. It is thus legal to possess codeine without a prescription, provided that it is compounded with at least one other active or inactive ingredient and that the dosage of each tablet, capsule, etc. does not exceed 100 mg or 2.5% concentration in the case of liquid preparations. The exemptions do not to apply to any preparation of codeine designed for injection.
United States
In the United States, codeine is regulated by the Controlled Substances Act. Federal law dictates that codeine be a Schedule II controlled substance when used in products for pain relief that contain codeine alone or more than 80 mg per dosage unit. Codeine without aspirin or acetaminophen (Tylenol) is very rarely available or prescribed to discourage abuse. Tablets of codeine in combination with aspirin or acetaminophen (paracetamol) and intended for pain relief are listed as Schedule III.
Cough syrups are classed as Schedule III, IV, or V, depending on formulation. For example, the acetaminophen/codeine antitussive liquid is a Schedule IV controlled substance.
Some states have chosen to reclassify codeine preparations at a more restrictive schedule to lower the instances of its abuse. Minnesota, for instance, has chosen to reclassify Schedule V some codeine preparations (e.g. Cheratussin) as a Schedule III controlled substance.
Schedule V controlled substances
Substances in this schedule have a low potential for abuse relative to substances listed in Schedule IV and consist primarily of preparations containing limited quantities of certain narcotics.
Examples of Schedule V substances include cough preparations containing not more than 200 milligrams of codeine per 100 milliliters or per 100 grams (Robitussin AC, Phenergan with Codeine).
References
- "Codeine Use During Pregnancy". Drugs.com. 3 February 2020. Archived from the original on 30 December 2019. Retrieved 7 February 2020.
- Bonewit-West K, Hunt SA, Applegate E (2012). Today's Medical Assistant: Clinical and Administrative Procedures. Elsevier Health Sciences. p. 571. ISBN 9781455701506. Archived from the original on 10 January 2023. Retrieved 20 August 2019.
- Polsten GR, Wallace MS (21 June 2016). "Analgesic Agents in Rheumatic Disease". In Firestein GS, Budd R, Gabriel SE, McInnes IB, O'Dell JR (eds.). Kelley and Firestein's Textbook of Rheumatology. Elsevier Health Sciences. pp. 1081–. ISBN 978-0-323-41494-4.
- ^ "Codeine". The American Society of Health-System Pharmacists. Archived from the original on 18 January 2016. Retrieved 5 January 2016.
- Shen H, He MM, Liu H, Wrighton SA, Wang L, Guo B, et al. (August 2007). "Comparative metabolic capabilities and inhibitory profiles of CYP2D6.1, CYP2D6.10, and CYP2D6.17". Drug Metabolism and Disposition. 35 (8): 1292–1300. doi:10.1124/dmd.107.015354. PMID 17470523. S2CID 2322678.
- Prommer E (2010). "Role of codeine in palliative care". Journal of Opioid Management. 7 (5): 401–406. doi:10.5055/jom.2011.0081. PMID 22165039.
- ^ Paul IM (February 2012). "Therapeutic options for acute cough due to upper respiratory infections in children". Lung. 190 (1): 41–44. doi:10.1007/s00408-011-9319-y. PMID 21892785. S2CID 23865647.
- Smith SM, Schroeder K, Fahey T (November 2014). "Over-the-counter (OTC) medications for acute cough in children and adults in community settings". The Cochrane Database of Systematic Reviews. 2014 (11): CD001831. doi:10.1002/14651858.CD001831.pub5. PMC 7061814. PMID 25420096.
- ^ Tobias JD, Green TP, Coté CJ (October 2016). "Codeine: Time to Say "No"". Pediatrics. 138 (4): e20162396. doi:10.1542/peds.2016-2396. PMID 27647717.
- ^ Newton D (2015). Prescription Drug Abuse: A Reference Handbook. ABC-CLIO. p. 20. ISBN 978-1-4408-3979-5. Archived from the original on 4 February 2017.
- Narcotic Drugs 2014 (PDF). International Narcotics Control Board. 2015. p. 21. ISBN 9789210481571. Archived (PDF) from the original on 2 June 2015.
- World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- Macleod AG, Ashford B, Voltz M, Williams B, Cramond T, Gorta L, et al. (June 2002). "Paracetamol versus paracetamol-codeine in the treatment of post-operative dental pain: a randomized, double-blind, prospective trial". Australian Dental Journal. 47 (2): 147–151. doi:10.1111/j.1834-7819.2002.tb00319.x. PMID 12139269.
- Straube C, Derry S, Jackson KC, Wiffen PJ, Bell RF, Strassels S, et al. (September 2014). "Codeine, alone and with paracetamol (acetaminophen), for cancer pain". The Cochrane Database of Systematic Reviews. 9 (9): CD006601. doi:10.1002/14651858.CD006601.pub4. PMC 6513650. PMID 25234029.
- Office of the Commissioner. "Press Announcements - FDA statement from Douglas Throckmorton, M.D., deputy center director for regulatory programs, Center for Drug Evaluation and Research, on new warnings about the use of codeine and tramadol in children & nursing mothers". www.fda.gov. Archived from the original on 20 April 2017. Retrieved 21 April 2017.
- McCrory DC, Coeytaux RR, Yancy WS Jr, Schmit KM, Kemper AR, Goode A, et al. (January 2013). "Assessment and Management of Chronic Cough". AHRQ Comparative Effectiveness Reviews. PMID 23367526.
- Guandalini S, Vaziri H (8 November 2010). Diarrhea: Diagnostic and Therapeutic Advances. New York, USA: Humana Press. p. 452. ISBN 978-1-60761-182-0. Archived from the original on 29 June 2023. Retrieved 9 October 2020.
- "Codeine – adverse effects". Medscape reference – Drugs, Diseases & Procedures. WebMD LLC. Archived from the original on 18 August 2012. Retrieved 27 September 2012.
- CTV News, Codeine use while breastfeeding may be dangerous Archived 1 September 2008 at the Wayback Machine, Wed. 20 August 2008
- ^ Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder SJ (August 2006). "Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother". Lancet. 368 (9536): 704. doi:10.1016/S0140-6736(06)69255-6. PMID 16920476. S2CID 46339578.
- "Safety review update of codeine use in children; new Boxed Warning and Contraindication on use after tonsillectomy and/or adenoidectomy". FDA. 20 February 2013. Archived from the original on 4 September 2014.
- "Opioids: Information for Health Professionals" (PDF). Alberta Health Services. Archived (PDF) from the original on 12 March 2017. Retrieved 9 March 2017.
- Fernandes LC, Kilicarslan T, Kaplan HL, Tyndale RF, Sellers EM, Romach MK (June 2002). "Treatment of codeine dependence with inhibitors of cytochrome P450 2D6". Journal of Clinical Psychopharmacology. 22 (3): 326–329. doi:10.1097/00004714-200206000-00014. PMID 12006904. S2CID 30804655.
- Kathiramalainathan K, Kaplan HL, Romach MK, Busto UE, Li NY, Säwe J, et al. (August 2000). "Inhibition of cytochrome P450 2D6 modifies codeine abuse liability". Journal of Clinical Psychopharmacology. 20 (4): 435–444. doi:10.1097/00004714-200008000-00008. PMID 10917405.
- Willmann S, Edginton AN, Coboeken K, Ahr G, Lippert J (December 2009). "Risk to the breast-fed neonate from codeine treatment to the mother: a quantitative mechanistic modeling study". Clinical Pharmacology and Therapeutics. 86 (6): 634–643. doi:10.1038/clpt.2009.151. PMID 19710640. S2CID 37771918.
- "Ireland on the verge of a codeine addiction epidemic, Irish Medical Journal warns". Irish Examiner. 14 March 2019. Archived from the original on 16 November 2022. Retrieved 16 November 2022.
- ^ Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, et al. (February 1994). "Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors". Molecular Pharmacology. 45 (2): 330–334. PMID 8114680. Archived from the original on 2 December 2020. Retrieved 22 June 2018.
- ^ Corbett AD, Paterson SJ, Kosterlitz HW (1993). "Selectivity of Ligands for Opioid Receptors". In Herz A, Akil H, Simon EJ (eds.). Opioids. Handbook of Experimental Pharmacology. Vol. 104 / 1. Springer Verlag. pp. 645–679. doi:10.1007/978-3-642-77460-7_26. ISBN 978-3-642-77462-1. ISSN 0171-2004.
- King TL, Miller EL (25 October 2010). "Analgesia and Anaesthesia". In King TL, Brucker MC (eds.). Pharmacology for Women's Health. Jones & Bartlett Publishers. pp. 332–. ISBN 978-1-4496-1073-9.
- Flood P, Aleshi P (28 February 2014). "Postoperative and Chronic Pain: Systematic and Regional Analgesia Techniques". In Chestnut DH, Wong CA, Tsen LC, Kee WD, Beilin Y, Mhyre J (eds.). Chestnut's Obstetric Anesthesia: Principles and Practice. Elsevier Health Sciences. pp. 611–. ISBN 978-0-323-11374-8. Archived from the original on 11 January 2023. Retrieved 22 June 2018.
- Tiziani AP (1 June 2013). Havard's Nursing Guide to Drugs. Elsevier Health Sciences. pp. 933–. ISBN 978-0-7295-8162-2. Archived from the original on 11 January 2023. Retrieved 22 June 2018.
- ^ Szucs-Reed RP, Gallagher RM (5 January 2012). "Chronic pain and opioids.". In Moore RJ (ed.). Handbook of Pain and Palliative Care: Biobehavioral Approaches for the Life Course. Springer Science & Business Media. pp. 499–. doi:10.1007/978-1-4419-1651-8_29. ISBN 978-1-4419-1651-8. S2CID 68670125.
- Neri C; Guama M; et al. Endogenous morphine and codeine in the brain of non human primate. Medical Science Montier 2004, 10, MS1-MS5.
- ^ Johnson JL, Rolan PE, Johnson ME, Bobrovskaya L, Williams DB, Johnson K, et al. (November 2014). "Codeine-induced hyperalgesia and allodynia: investigating the role of glial activation". Translational Psychiatry. 4 (11): e482. doi:10.1038/tp.2014.121. PMC 4259992. PMID 25386959.
- ^ Papich MG (1 January 2016). "Codeine". Saunders Handbook of Veterinary Drugs (fourth ed.). W.B. Saunders. pp. 183–184. doi:10.1016/b978-0-323-24485-5.00175-3. ISBN 978-0-323-24485-5.
- Hitchings A, Lonsdale D, Burrage D, Baker E (2015). Top 100 drugs: clinical pharmacology and practical prescribing. Churchill Livingstone. p. 168. ISBN 978-0-7020-5516-4.
- ^ Stefano GB, Ptáček R, Kuželová H, Kream RM (2012). "Endogenous morphine: up-to-date review 2011" (PDF). Folia Biologica. 58 (2): 49–56. doi:10.14712/fb2012058020049. PMID 22578954. Archived (PDF) from the original on 24 August 2016.
Positive evolutionary pressure has apparently preserved the ability to synthesize chemically authentic morphine, albeit in homeopathic concentrations, throughout animal phyla.
- Srinivasan V, Wielbo D, Tebbett IR (1997). "Analgesic effects of codeine-6-glucuronide after intravenous administration". European Journal of Pain. 1 (3): 185–190. doi:10.1016/S1090-3801(97)90103-8. PMID 15102399. S2CID 23099329.
- Lurcott G (1998). "The effects of the genetic absence and inhibition of CYP2D6 on the metabolism of codeine and its derivatives, hydrocodone and oxycodone". Anesthesia Progress. 45 (4): 154–156. PMC 2148980. PMID 10483388.
- Gasche Y, Daali Y, Fathi M, Chiappe A, Cottini S, Dayer P, et al. (December 2004). "Codeine intoxication associated with ultrarapid CYP2D6 metabolism". The New England Journal of Medicine. 351 (27): 2827–2831. doi:10.1056/NEJMoa041888. PMID 15625333.
- Caraco Y (December 2004). "Genes and the response to drugs". The New England Journal of Medicine. 351 (27): 2867–2869. doi:10.1056/NEJMe048278. PMID 15625340.
- Gardiner SJ, Begg EJ (September 2006). "Pharmacogenetics, drug-metabolizing enzymes, and clinical practice". Pharmacological Reviews. 58 (3): 521–590. doi:10.1124/pr.58.3.6. PMID 16968950. S2CID 25747320.
- Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE, Caudle KE, et al. (April 2014). "Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update". Clinical Pharmacology and Therapeutics. 95 (4): 376–382. doi:10.1038/clpt.2013.254. PMC 3975212. PMID 24458010.
- The Merck Index, 13th Edition: Morphine Hydrochloride
- Cone EJ, Darwin WD, Gorodetzky CW (May 1979). "Comparative metabolism of codeine in man, rat, dog, guinea-pig and rabbit: identification of four new metabolites". The Journal of Pharmacy and Pharmacology. 31 (5): 314–317. doi:10.1111/j.2042-7158.1979.tb13507.x. PMID 37301. S2CID 42128300.
- "Codeine Phosphate Tablets 30mg - Summary of Product Characteristics (SmPC) - (emc)". www.medicines.org.uk. Archived from the original on 23 January 2022. Retrieved 29 December 2019.
- "Spotlight on Codeine".
- ^ "Unlocking the opium poppy's biggest secret: Genes that make codeine, morphine". ScienceDaily. Archived from the original on 9 September 2017. Retrieved 29 December 2019.
- "Virtual ChemBook". chemistry.elmhurst.edu. Archived from the original on 29 July 2003. Retrieved 29 December 2019.
- US expired 6204337, Robert C. Corcoran & Junning Ma, "Solid-phase synthesis of codeine from morphine", published 2001-03-20, issued 2001-03-20, assigned to University and Community College System of Nevada UCCSN
- "How Johnson & Johnson companies used a 'super poppy' to make narcotics for America's most abused opioid pills". The Washington Post. Archived from the original on 15 May 2022. Retrieved 20 April 2022.
- Report of Committee on drug addiction, 1929–1941. National Research Council (US).
- Hayes AN, Gilbert SG (2009). "Historical milestones and discoveries that shaped the toxicology sciences". In Luch A (ed.). Molecular, clinical and environmental toxicology. Springer. p. 20. ISBN 978-3-7643-8335-0.
- ^ Wisniak J (1 March 2013). "Pierre-Jean Robiquet". Educación Química. 24: 139–149. doi:10.1016/S0187-893X(13)72507-2. ISSN 0187-893X.
- Li G, Lou M, Qi X (2022). "A brief overview of classical natural product drug synthesis and bioactivity". Organic Chemistry Frontiers. 9 (2): 517–571. doi:10.1039/d1qo01341f. ISSN 2052-4129. S2CID 244465703.
- Lindsey J, Barnes WH (April 1955). "The Crystal and Molecular Structure of Codeine Hydrobromide Dihydrate". Acta Crystallographica. 8 (4): 227. Bibcode:1955AcCry...8..227L. doi:10.1107/S0365110X55000753.
- Fraser TR (January 1889). "The Relative Value of Opium, Morphine, and Codeine in Diabetes Mellitus". British Medical Journal. 1 (1464): 118–119. doi:10.1136/bmj.1.1464.118. PMC 2154578. PMID 20752554.
- The American Practitioner. 1912. Archived from the original on 29 June 2023. Retrieved 9 October 2020.
- DEA, ibid
- Merck Index
- Anonymous Codeine. In The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals; O'Neil, M. J., Ed.; Merck: Whitehouse Station, NJ, 2006; pp 2417.
- van Solinge TB (1996). "7. La politique de soins des années quatre-vingt-dix". L'héroïne, la cocaïne et le crack en France. Trafic, usage et politique (in French). Amsterdam: CEDRO Centrum voor Drugsonderzoek, Universiteit van Amsterdam. pp. 247–262. Archived from the original on 25 February 2021. Retrieved 26 April 2006.
- Leinwand D (18 October 2006). "DEA warns of soft drink-cough syrup mix". USA Today. Archived from the original on 28 November 2006. Retrieved 23 October 2006.
- "Pimp C's death caused by overdose and sleep condition – Houston Chronicle". Chron.com. 4 February 2008. Archived from the original on 31 December 2013. Retrieved 12 January 2014.
- Hogshire J (June 1999). Pills-A-Go-Go: A Fiendish Investigation into Pill Marketing, Art, History & Consumption. Los Angeles: Feral House. pp. 216–223. ISBN 978-0-922915-53-8.
- Savchuk SA, Barsegyan SS, Barsegyan IB, Kolesov GM (2011). "Chromatographic study of expert and biological samples containing desomorphine". Journal of Analytical Chemistry. 63 (4): 361–70. doi:10.1134/S1061934808040096. S2CID 54546428.
- Thevis M, Opfermann G, Schänzer W (2003). "Urinary concentrations of morphine and codeine after consumption of poppy seeds". Journal of Analytical Toxicology. 27 (1): 53–56. doi:10.1093/jat/27.1.53. PMID 12587685.
- Cone EJ, Welch P, Paul BD, Mitchell JM (1991). "Forensic drug testing for opiates, III. Urinary excretion rates of morphine and codeine following codeine administration". Journal of Analytical Toxicology. 15 (4): 161–166. doi:10.1093/jat/15.4.161. PMID 1943064.
- Baselt R (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City CA: Biomedical Publications. pp. 355–360.
- International Narcotics Control Board. "List of Narcotic Drugs under International Control" (PDF). Archived from the original (PDF) on 10 May 2012. Retrieved 24 May 2006.
- ^ Foley M, Harris R, Rich E, Rapca A, Bergin M, Norman I, et al. (November 2015). "The availability of over-the-counter codeine medicines across the European Union". Public Health. 129 (11): 1465–1470. doi:10.1016/j.puhe.2015.06.014. PMID 26215740. Archived from the original on 9 July 2020. Retrieved 8 July 2020.
- "Over-the-counter Codeine: The End Of An Era, And Preparing For The Changes Ahead". Australian and New Zealand Mental Health Association, and HealtheCare. Archived from the original on 11 July 2021. Retrieved 11 July 2021.
- "Painkillers with codeine won't be available over the counter from 2018". ABC.net.au. 19 December 2016. Archived from the original on 21 December 2016. Retrieved 20 December 2016.
- McCoy J, Bruno R, Nielsen S (February 2018). "Attitudes in Australia on the upscheduling of over-the-counter codeine to a prescription-only medication". Drug and Alcohol Review. 37 (2): 257–261. doi:10.1111/dar.12568. PMID 28597531. S2CID 26296460.
- ^ "Letter to Office of Legislative and Regulatory Affairs - Health Canada" (PDF). Canadian Pharmacists Association. Archived (PDF) from the original on 22 September 2020. Retrieved 22 August 2019.
- Chao YS, Severn M. "Non-prescription Analgesic and Antitussive Medications Containing Codeine: A Review of Clinical Effectiveness and Safety" (PDF). CADTH Canadian Agency for Drugs and Technologies in Health. Archived (PDF) from the original on 26 September 2020. Retrieved 20 June 2018.
- Legislative Services Branch (19 September 2019). "Consolidated Federal Laws of Canada, Controlled Drugs and Substances Act". Justice Laws Website. Archived from the original on 23 September 2020. Retrieved 27 September 2020.
- "College of Pharmacists of Manitoba Newsletter Summer 2018" (PDF). CPHM. Archived from the original (PDF) on 21 July 2020. Retrieved 22 August 2019.
- "Tylenol 1 to require prescription in Manitoba in February 2016". www.cbc.ca. Archived from the original on 31 May 2016. Retrieved 4 June 2016.
- Taylor S. "'Opioid epidemic:' Pharmacists want to restrict low-dose codeine products like Tylenol 1". National Post. Archived from the original on 28 August 2021. Retrieved 22 August 2019.
- "Over-the-counter codeine should be limited in wake of 'opioid epidemic', says Sask. pharmacist". CBC. Archived from the original on 24 October 2020. Retrieved 22 August 2019.
- "Recalls and Safety Alerts". Government of Canada. 25 August 2021. Archived from the original on 5 November 2020. Retrieved 22 August 2019.
- Foley M, Breindahl T, Hindersson P, Deluca P, Kimergård A (January 2016). "Misuse of 'over-the-counter' codeine analgesics: does formulation play a role?". Public Health. 130: 95–96. doi:10.1016/j.puhe.2015.10.006. PMID 26612458.
- Koppel K (23 November 2022). "Pharmacists welcome ban on OTC sale of painkillers containing codeine". ERR. Retrieved 8 November 2023.
- "vital list of codein". vidal.com. Archived from the original on 21 July 2011. Retrieved 12 January 2011.
- Wilkes D. "OTCToolbox - France restricts dextromethorphan and codeine". www.otctoolbox.com. Archived from the original on 16 January 2019. Retrieved 28 October 2017.
- "Pharmacies in Greece". About.com. Archived from the original on 6 October 2010. Retrieved 10 October 2009.
- ^ Laws of Hong Kong, Dangerous Drugs Ordinance, Chapter 134 "Hong Kong e-Legislation". Archived from the original on 16 October 2012. Retrieved 16 October 2016.
- Mudgill A (14 March 2016). "Pfizer, Abbott shares tank after ban on Corex, Phensedyl and 327 other drugs – The Economic Times". The Economic Times. Archived from the original on 17 November 2016. Retrieved 14 March 2016.
- "Codeine regulations cause a few headaches". The Irish Times. 8 August 2010. Archived from the original on 3 September 2010.
- Office of The Attorney General, Regulation 5(1)(e) of the Regulation of Retail Pharmacy Businesses Regulations 2008 (S.I. 488 of 2008) Archived 27 July 2011 at the Wayback Machine
- "Non-Prescription Medicinal Products Containing Codeine: Draft Guidance for Pharmacists". Pharmaceutical Society of Ireland. Archived from the original on 21 May 2013.
- "Tachidol - Foglio Illustrativo". www.my-personaltrainer.it (in Italian). Archived from the original on 3 February 2022. Retrieved 3 February 2022.
- Aneton cough medicine package.
- ^ "Nigeria bans all codeine cough syrup". BBC News Online. 1 May 2018. Archived from the original on 2 May 2018. Retrieved 3 May 2018.
- "SA's silent codeine addiction uncovered | IOL News". Archived from the original on 17 October 2016. Retrieved 16 October 2016.
- "Misuse of Drugs Act 1971". Legislation.gov.uk. Archived from the original on 8 November 2013. Retrieved 12 January 2014.
- "List of most commonly encountered drugs currently controlled under the misuse of drugs legislation". GOV.UK. 20 October 2016. Archived from the original on 8 December 2019. Retrieved 7 February 2020.
- "Misuse of Drugs Act 1971". Legislation.gov.uk. Archived from the original on 28 January 2014. Retrieved 12 January 2014.
- "The Misuse of Drugs Regulations 2001". Legislation.gov.uk. 9 December 2013. Archived from the original on 29 January 2014. Retrieved 12 January 2014.
- Valeant Pharmaceuticals. "Prescribing Information for Capital with Codeine (paracetamol with codeine) showing Schedule V designation" (PDF). Archived from the original (PDF) on 17 July 2011. Retrieved 25 February 2011.
- "152.02 Schedules Of Controlled Substances". State of Minnesota. Archived from the original on 30 July 2013. Retrieved 30 May 2013.
- Controlled Substance Schedules. Archived 21 November 2020 at the Wayback Machine U.S. DEPARTMENT OF JUSTICE, DRUG ENFORCEMENT ADMINISTRATION: Diversion Control Division.
Notes
- S4 only if in drug combinations; see #Legal status.
- Class C1 only for low doses; see exemptions on Portaria SVS/MS 344/98.
- Pharmacy medicine if purchased in a low dose from a licensed pharmacy or in low dose drug combination; see #Legal status.
- Schedule III-V only if in drug combination; see #Legal status.
- Schedule III only if in drug combination; see #Legal status.
Further reading
- Dean L (2012). "Codeine Therapy and CYP2D6 Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 28520350. Bookshelf ID: NBK100662.
External links
| Antidiarrheals, intestinal anti-inflammatory and anti-infective agents (A07) | |
|---|---|
| Rehydration | |
| Intestinal anti-infectives | |
| Intestinal adsorbents |
|
| Antipropulsives (opioids) |
|
| Intestinal anti-inflammatory agents |
|
| Antidiarrheal micro-organisms | |
| Other antidiarrheals | |
| |
| Cough and cold preparations (R05) | |||||
|---|---|---|---|---|---|
| Expectorants | |||||
| Mucolytics | |||||
| Cough suppressants |
| ||||
| |||||
| Opioid receptor modulators | |||||
|---|---|---|---|---|---|
| μ-opioid (MOR) |
| ||||
| δ-opioid (DOR) |
| ||||
| κ-opioid (KOR) |
| ||||
| Nociceptin (NOP) |
| ||||
| Others |
| ||||
| Glycine receptor modulators | |||||
|---|---|---|---|---|---|
| Receptor (ligands) |
| ||||
| Transporter (blockers) |
| ||||