| Revision as of 08:43, 5 February 2010 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits update← Previous edit | Latest revision as of 07:36, 18 October 2024 edit undoChristian75 (talk | contribs)Extended confirmed users, New page reviewers, Pending changes reviewers, Rollbackers114,301 edits Undid revision 1249174559 by 47.229.144.175 (talk) Unexplained. An enoate is salt or ester of a carboxylic acid. Enolate is a salt of the enol form of a tautomeric aldehyde or ketoneTag: Undo | ||
| (217 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{short description|Chemical coupling reaction}} | |||
| {{distinguish|Wittig rearrangement (disambiguation){{!}}Wittig rearrangement}} | |||
| {{Use dmy dates|date=April 2023}} | |||
| {{Reactionbox | {{Reactionbox | ||
| | Verifiedfields = changed | |||
| | Reference = | |||
| | verifiedrevid = 342064940 | |||
| | Type = Carbon-carbon bond forming reaction | |||
| | Reference = | |||
| | Type = Coupling reaction | |||
| | NamedAfter = ] | | NamedAfter = ] | ||
| | Section3 = {{Reactionbox Identifiers | | Section3 = {{Reactionbox Identifiers | ||
| | RSC_ontology_id_verified = |
| RSC_ontology_id_verified = rsccite | ||
| | RSC_ontology_id = 0000015 | | RSC_ontology_id = 0000015 | ||
| | OrganicChemistryNamed = wittig-reaction | | OrganicChemistryNamed = wittig-reaction | ||
| | MarchID = |
| MarchID = 16–44 | ||
| | MarchEdition = 6th | | MarchEdition = 6th | ||
| | MarchPage = |
| MarchPage = | ||
| }} | }} | ||
| | Section1 = {{Reactionbox Conditions | | Section1 = {{Reactionbox Conditions | ||
| Line 16: | Line 21: | ||
| | Reaction = {{Reactionbox Reaction | | Reaction = {{Reactionbox Reaction | ||
| | Reactant1 = ] or ] | | Reactant1 = ] or ] | ||
| | Reactant2 = triphenyl phosphonium ylide | | Reactant2 = ] | ||
| | Reagent1 = strong ] | |||
| | Product1 = ] | | Product1 = ] | ||
| | Sideproduct1 = ] | | Sideproduct1 = ] | ||
| }} | }} | ||
| }} | }} | ||
| The '''Wittig Reaction''' is a ] of an ] or ] with a triphenyl ] (often called a '''Wittig reagent''') to give an ] and ].<ref>{{cite journal | |||
| | author = ], ] | |||
| | journal =] | |||
| | year = 1954 | |||
| | volume = 87 | |||
| | pages = 1318 | |||
| | title = Über Triphenyl-phosphin-methylene als olefinbildende Reagenzien I | |||
| | doi = 10.1002/cber.19540870919}}</ref><ref>{{cite journal | |||
| | author = Georg Wittig, Werner Haag | |||
| | journal =] | |||
| | year = 1955 | |||
| | volume = 88 | |||
| | pages = 1654–1666 | |||
| | title = Über Triphenyl-phosphin-methylene als olefinbildende Reagenzien II | |||
| | doi = 10.1002/cber.19550881110}}</ref> | |||
| ] | |||
| The Wittig reaction was discovered in 1954 by ], for which he was awarded the ] in 1979. It is widely used in ] for the preparation of alkenes.<ref>Maercker, A. ''Org. React.'' '''1965''', ''14'', 270-490. (Review)</ref><ref>W. Carruthers, ''Some Modern Methods of Organic Synthesis'', Cambridge University Press, Cambridge, UK, 1971, pp81-90. (ISBN 0-521-31117-9)</ref><ref>{{cite journal | author = R. W. Hoffmann | title = Wittig and His Accomplishments: Still Relevant Beyond His 100th Birthday | year = 2001 | journal = ] | volume = 40 | issue = 8 | pages = 1411–1416 | doi = 10.1002/1521-3773(20010417)40:8<1411::AID-ANIE1411>3.0.CO;2-U}}</ref> It should not be confused with the ]. | |||
| The '''Wittig reaction''' or Wittig olefination is a ] of an ] or ] with a triphenyl phosphonium ] called a ]. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes.<ref>Maercker, A. ''Org. React.'' '''1965''', ''14'', 270–490.</ref><ref>W. Carruthers, ''Some Modern Methods of Organic Synthesis'', Cambridge University Press, Cambridge, UK, 1971, 81–90. ({{ISBN|0-521-31117-9}})</ref><ref>{{Cite journal| author = R. W. Hoffmann | title = Wittig and His Accomplishments: Still Relevant Beyond His 100th Birthday | year = 2001 | journal = ] | volume = 40 | issue = 8 | pages = 1411–1416 | doi = 10.1002/1521-3773(20010417)40:8<1411::AID-ANIE1411>3.0.CO;2-U | pmid=11317288}}</ref> Most often, the Wittig reaction is used to introduce a ] using ] (Ph<sub>3</sub>P=CH<sub>2</sub>). Using this reagent, even a sterically hindered ketone such as ] can be converted to its methylene derivative. | |||
| Wittig reactions are most commonly used to couple aldehydes and ketones to singly substituted phosphine ]s. With simple ylides this results in almost exclusively the ] product. In order to obtain the E-alkene, the ] of the Wittig reaction can be performed. | |||
| ] | |||
| ==Reaction mechanism== | ==Reaction mechanism== | ||
| Mechanistic studies have focused on unstabilized ylides, because the intermediates can be followed by ]. The existence and interconversion of the betaine ('''3a''' and '''3b''') is subject of ongoing research.<ref>{{Cite journal|author1=E. Vedejs |author2=C. F. Marth |name-list-style=amp | title = Mechanism of Wittig reaction: evidence against betaine intermediates | year = 1990 | journal = ] | volume = 112 | issue = 10 | pages = 3905–3909 | doi=10.1021/ja00166a026}}</ref> For lithium-free Wittig reactions, studies support a concerted formation of the ] without intervention of a betaine. In particular, phosphonium ylides '''1''' react with carbonyl compounds '''2''' via a ] that is sometimes described as having topology to directly form the oxaphosphetanes '''4a''' and '''4b'''. Under lithium-free conditions, the ] of the product '''5''' is due to the kinetically controlled addition of the ylide '''1''' to the carbonyl '''2'''. When lithium is present, there may be ] of the intermediates, possibly via betaine species '''3a''' and '''3b'''.<ref>], A. B. Reitz, M. S. Mutter, R. R. Inners, and H. R. Almond, Jr., "Detailed Rate Studies on the Wittig Reaction of Non-Stabilized Phosphorus Ylides via <sup>31</sup>P, <sup>1</sup>H, and <sup>13</sup>C NMR Spectroscopy. Insight into Kinetic vs. Thermodynamic Control of Stereochemistry", J. Am. Chem. Soc., '''107''', 1068–1070 (1985)</ref><ref>Bruce E. Maryanoff, A. B. Reitz, D. W. Graden, and H. R. Almond, Jr., "NMR Rate Study on the Wittig Reaction of 2,2-Dimethylpropanal and Tributylbutylidene-phosphorane", Tetrahedron Lett., '''30''', 1361–1364 (1989)</ref><ref>Bruce E. Maryanoff, A. B. Reitz, M. S. Mutter, R. R. Inners, H. R. Almond, Jr., R. R. Whittle, and R. A. Olofson, "Stereochemistry and Mechanism of the Wittig Reaction. Diastereomeric Reaction Intermediates and Analysis of the Reaction Course", J. Am. Chem. Soc., '''108''', 7664–7678 (1986)</ref> ] and A. B. Reitz identified the issue about equilibration of Wittig intermediates and termed the process "stereochemical drift". For many years, the stereochemistry of the Wittig reaction, in terms of carbon-carbon bond formation, had been assumed to correspond directly with the Z/E stereochemistry of the alkene products. However, certain reactants do not follow this simple pattern. ] salts can also exert a profound effect on the stereochemical outcome.<ref>A. B. Reitz, S. O. Nortey, A. D. Jordan, Jr., M. S. Mutter, and Bruce E. Maryanoff, "Dramatic Concentration Dependence of Stereochemistry in the Wittig Reaction. Examination of the Lithium-Salt Effect", J. Org. Chem., '''51''', 3302–3308 (1986)</ref> | |||
| ===Classical mechanism=== | |||
| The steric bulk of the ] '''1''' influences the stereochemical outcome of ] to give a predominance of the ] '''3''' (c.f. ]). Note that for betaine '''3''' both R<sub>1</sub> and R<sub>2</sub> as well as PPh3+ and O- are positioned anti (trans-diaxial) to one another. | |||
| ] | |||
| Carbon-carbon bond rotation gives the betaine '''4''', which then forms the oxaphosphetane '''5'''. Elimination gives the desired Z-alkene '''6''' and ] '''7'''. With simple Wittig reagents, the first step occurs easily with both ]s and ]s, and the decomposition of the betaine (to form '''5''') is the ]. However with ] (where R<sub>1</sub> stabilises the negative charge) the first step is the slowest step, so the overall rate of alkene formation decreases and a bigger proportion of the alkene product is the ]. This also explains why stabilised reagents fail to react well with ]. | |||
| Mechanisms differ for ] and ] ]s and for ] and ] phosphonium ylides. Evidence suggests that the Wittig reaction of ] aldehydes under lithium-salt-free conditions do not equilibrate and are therefore under ].<ref>{{Cite journal| author = E. Vedejs, C. F. Marth and R. Ruggeri | title = Substituent effects and the Wittig mechanism: the case of stereospecific oxaphosphetane decomposition | year = 1988 | journal = ] | volume = 110 | issue = 12 | pages = 3940–48 | doi = 10.1021/ja00220a036}}</ref><ref>{{Cite journal|author1=E. Vedejs|author2=C. F. Marth|name-list-style=amp | title = Mechanism of the Wittig reaction: the role of substituents at phosphorus | year = 1988 | journal = J. Am. Chem. Soc. | volume = 110 | issue = 12 | pages = 3948–3958 | doi = 10.1021/ja00220a037}}</ref> ] has put forth a theory to explain the stereoselectivity of stabilized and unstabilized Wittig reactions.<ref>Vedejs, E.; Peterson, M. J. ''Top. Stereochem.'' '''1994''', ''21'', 1.</ref> | |||
| ] | |||
| Strong evidence indicated that under Li-free conditions, Wittig reactions involving unstabilized (R<sub>1</sub>= alkyl, H), semistabilized (R<sub>1</sub> = aryl), and stabilized (R<sub>1</sub> = EWG) Wittig reagents all proceed via a /retro- mechanism under kinetic control, with oxaphosphetane as the one and only intermediate.<ref>{{Cite journal|last1=Byrne|first1=Peter A.|last2=Gilheany|first2=Declan G.|date=2013|title=The modern interpretation of the Wittig reaction mechanism|journal=Chemical Society Reviews|language=en|volume=42|issue=16|pages=6670–96|doi=10.1039/c3cs60105f|pmid=23673458|issn=0306-0012|hdl=10197/4939|hdl-access=free}}</ref> | |||
| ===Recent developments=== | |||
| Recent research has shown that the ] presented above does not account for all experimental results. Mechanistic studies have been done mostly on unstablilized ylides, because the intermediates can be followed by ]. The existence and interconversion of the betaine ('''3a''' and '''3b''') is still under debate and a subject of ongoing research.<ref>{{cite journal | author = E. Vedejs and C. F. Marth | title = Mechanism of Wittig reaction: evidence against betaine intermediates | year = 1990 | journal = ] | volume = 112 | issue = 10 | pages = 3905–3909 | doi=10.1021/ja00166a026}}</ref> There is evidence that phosphonium ylides '''1''' can react with carbonyl compounds '''2''' via a π²s/π²a ] to directly form the oxaphosphatanes '''4a''' and '''4b'''. The ] of the product '''5''' is due to the addition of the ylide '''1''' to the carbonyl '''2''' and to the ability of the intermediates to ].<ref>], A. B. Reitz, M. S. Mutter, R. R. Inners, and H. R. Almond, Jr., "Detailed Rate Studies on the Wittig Reaction of Non-Stabilized Phosphorus Ylides via <sup>31</sup>P, <sup>1</sup>H, and <sup>13</sup>C NMR Spectroscopy. Insight into Kinetic vs. Thermodynamic Control of Stereochemistry", J. Am. Chem. Soc., '''107''', 1068-1070 (1985)</ref><ref>], A. B. Reitz, D. W. Graden, and H. R. Almond, Jr., "NMR Rate Study on the Wittig Reaction of 2,2-Dimethylpropanal and Tributylbutylidene-phosphorane", Tetrahedron Lett., '''30''', 1361-1364 (1989)</ref><ref>], A. B. Reitz, M. S. Mutter, R. R. Inners, H. R. Almond, Jr., R. R. Whittle, and R. A. Olofson, "Stereochemistry and Mechanism of the Wittig Reaction. Diastereomeric Reaction Intermediates and Analysis of the Reaction Course", J. Am. Chem. Soc., '''108''', 7664-7678 (1986)</ref> ] and Reitz identified the issue about equilibration of Wittig intermediates and termed the process "stereochemical drift". For many years, the stereochemistry of the Wittig reaction, in terms of carbon-carbon bond formation, had been assumed to correspond directly with the Z/E stereochemistry of the alkene products. However, certain reactants do not follow this simple pattern. ] salts can also exert a profound effect on the stereochemical outcome.<ref>A. B. Reitz, S. O. Nortey, A. D. Jordan, Jr., M. S. Mutter, and ], "Dramatic Concentration Dependence of Stereochemistry in the Wittig Reaction. Examination of the Lithium-Salt Effect", J. Org. Chem., '''51''', 3302-3308 (1986)</ref> | |||
| ] | |||
| There are distinct differences in the mechanisms of ] and ] ]s and of ] and ] phosphonium ylides. Vedejs ''et al.'' have provided evidence that the Wittig reaction of ] aldehydes under lithium-salt-free conditions do not equilibrate and are therefore under ].<ref>{{cite journal | author = E. Vedejs, C. F. Marth and R. Ruggeri | title = Substituent effects and the Wittig mechanism: the case of stereospecific oxaphosphetane decomposition | year = 1988 | journal = ] | volume = 110 | issue = 12 | pages = 3940–3948 | doi = 10.1021/ja00220a036}}</ref><ref>{{cite journal | author = E. Vedejs and C. F. Marth | title = Mechanism of the Wittig reaction: the role of substituents at phosphorus | year = 1988 | journal = ] | volume = 110 | issue = 12 | pages = 3948–3958 | doi = 10.1021/ja00220a037}}</ref> Vedejs has put forth a theory to explain the stereoselectivity of stabilized and unstabilized Wittig reactions.<ref>Vedejs, E.; Peterson, M. J. ''Top. Stereochem.'' '''1994''', ''21'', 1.</ref> | |||
| ==Wittig reagents== | |||
| === Preparation of simple ylides=== | |||
| The Wittig reagent is usually prepared from a ], which is in turn made by the reaction of ] with an ]. To form the Wittig reagent (ylide), the phosphonium salt is suspended in a solvent such as ] or ] and a strong base such as ] or ] is added. | |||
| Ph<sub>3</sub>P<sup>+</sup>−CH<sub>2</sub>−R X<sup>−</sup> + ] → Ph<sub>3</sub>P=CH−R + LiX + ] | |||
| The simplest ylide used is methylenetriphenylphosphorane (Ph<sub>3</sub>P<sup>+</sup>−C<sup>−</sup>H<sub>2</sub>), and this is also the basis of an alternative synthesis of Wittig reagents. Substituted ylides can be made by alkylation of Ph<sub>3</sub>P=CH<sub>2</sub> with a primary ] R−CH<sub>2</sub>−X, to produce a substituted phosphonium salt: | |||
| Ph<sub>3</sub>P=CH<sub>2</sub> + ] → Ph<sub>3</sub>P<sup>+</sup>−CH<sub>2</sub>− CH<sub>2</sub>−R X<sup>−</sup> | |||
| which can be ] with C<sub>4</sub>H<sub>9</sub>Li to make Ph<sub>3</sub>P=CH−CH<sub>2</sub>−R. | |||
| ===Structure of the ylide=== | |||
| The Wittig reagent may be written in the '''phosphorane''' form (the more familiar representation) or the '''ylide''' form: | |||
| ] | |||
| However the phosphorane resonance requires expansion of the ] on phosphorus. This ] cannot (yet) be explained well in terms of standard bonding theory, and this resonance is rather less favoured than the more familiar p–p overlap seen in π-bonded compounds as ]s or ]s. This means that the ylide form is a significant contributor, and the carbon is quite ]. | |||
| The phosphorane obviously can accommodate the extra pair of electrons by using its vacant d-orbitals. | |||
| ===Reactivity=== | |||
| Simple phosphoranes are very reactive and are unstable in the presence of air of moisture. They are therefore prepared in a scrupulously dry solvent (usually THF) under nitrogen or argon and the carbonyl compound is added as soon as the phosphorane has been formed. | |||
| More stable phosphoranes are obtained when the ylide contains a group that can stabilise the negative charge from the ]. | |||
| For example: Ph<sub>3</sub>P=CH–COOR, Ph<sub>3</sub>P=CH–Ph. ] | |||
| These are formed more readily, requiring treatment of the phosphonium salt only with NaOH, and the are usually isolable, ] compounds. | |||
| These are less reactive than simple ylides, and so they usually fail to react with ketones, necessitating the use of the ] reaction as an alternative. | |||
| They can be prepared from the phosphonium salts using weaker bases than butyllithium such as ]s and (in some cases) ]. They usually give rise to an E-alkene product when they react, rather than the more usual Z-alkene. | |||
| ==Scope and limitations== | ==Scope and limitations== | ||
| ===Functional group tolerance=== | |||
| The Wittig reaction has become a popular method for ] synthesis precisely because of its wide applicability. Unlike ]s (such as ] of ]s), which produce mixtures of alkene ]s determined by ], the Wittig reaction forms the double bond in one position with no ambiguity. | |||
| The Wittig reagents generally tolerate ] compounds containing several kinds of functional groups such as ], ], ], ]s, and sometimes ] and ]s.<ref>Smith (2020), ''March's Organic Chemistry'', rxn. 16-44.</ref> Even ], ], and ] groups can be present if ] with the ylide — these are the ] mentioned above. Bis-ylides (containing two P=C bonds) have also been made and used successfully.<ref>{{Cite journal|author1=B. E. Maryanoff |author2=A. B. Reitz |name-list-style=amp | title = The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects | year = 1989 | journal = ] | volume = 89 | issue = 4 | pages = 863–927 | doi = 10.1021/cr00094a007}}</ref> There can be a problem with ] ketones, where the reaction may be slow and give poor yields, particularly with stabilized ylides, and in such cases the ] (using phosphonate esters) is preferred. Another reported limitation is the often labile nature of ]s, which can oxidize, polymerize or decompose. In a so-called tandem oxidation-Wittig process the aldehyde is formed ] by oxidation of the corresponding alcohol.<ref>{{OrgSynth | author = Richard J. K. Taylor, Leonie Campbell, and Graeme D. McAllister | title = (±) trans-3,3'-(1,2-Cyclopropanediyl)bis-2-(E)-propenoic Acid, Diethyl Ester: Tandem Oxidation Procedure (TOP) using MnO<sub>2</sub> Oxidation-Stabilized Phosphorane Trapping | year = 2008 | volume = 85 | pages = 15–26 | url = http://www.orgsyn.org/orgsyn/pdfs/V85P0015.pdf}}</ref> | |||
| === Stereochemistry === | |||
| A large variety of ]s and ]s are effective in the reaction, though ] derivatives such as ]s fail to react usefully. Thus mono-, di- and trisubstituted alkenes can all be prepared in good yield in most cases. The ] compound can tolerate several groups such as ], ], aromatic ] and even ester groups. There can be a problem with ] ketones, where the reaction may be slow and give poor yields, particularly with stabilised ylides, and in such cases the ] (using phosphonate esters) is preferred. Another reported limitation is the often labile nature of ]s which can oxidize, polymerize or decompose. In a so-called Tandem Oxidation-Wittig Process the aldehyde is formed ] by oxidation of the corresponding ].<ref>{{OrgSynth | author = Richard J. K. Taylor, Leonie Campbell, and Graeme D. McAllister | title = (±) trans-3,3'-(1,2-Cyclopropanediyl)bis-2-(E)-propenoic Acid, Diethyl Ester: Tandem Oxidation Procedure (TOP) using MnO<sub>2</sub> Oxidation-Stabilized Phosphorane Trapping | year = 2008 | volume = 85 | pages = 15-26 | url = http://www.orgsyn.org/orgsyn/pdfs/V85P0015.pdf}}</ref> | |||
| For the reaction with aldehydes, the double bond geometry is readily predicted based on the nature of the ylide. With unstabilised ylides (R<sup>3</sup> = alkyl) this results in ] product with moderate to high selectivity. If the reaction is performed in ] in the presence of ] or ], the product is almost exclusively the Z-isomer.<ref>{{Cite journal |author1=L. D. Bergelson |author2=M. M. Shemyakin |name-list-style=amp |year=1964 |title=Synthesis of Naturally Occurring Unsaturated Fatty Acids by Sterically Controlled Carbonyl Olefination |journal=] |volume=3 |issue=4 |pages=250–260 |doi=10.1002/anie.196402501}}</ref> With stabilized ylides (R<sup>3</sup> = ester or ketone), the (''E'')-alkene is formed with high selectivity. The (''E'')/(''Z'') selectivity is often poor with semistabilized ylides (R<sup>3</sup> = aryl).<ref>{{Cite journal |last1=Robiette |first1=Raphaël |last2=Richardson |first2=Jeffery |last3=Aggarwal |first3=Varinder K. |last4=Harvey |first4=Jeremy N. |date=2006-02-01 |title=Reactivity and Selectivity in the Wittig Reaction: A Computational Study |journal=Journal of the American Chemical Society |volume=128 |issue=7 |pages=2394–2409 |doi=10.1021/ja056650q |issn=0002-7863 |pmid=16478195}}</ref> | |||
| To obtain the (''E'')-alkene for unstabilized ylides, the Schlosser modification of the Wittig reaction can be used. Alternatively, the ] and its variants also provide the (''E'')-alkene selectively. Ordinarily, the ] provides the (''E'')-enoate (α,β-unsaturated ester), just as the Wittig reaction does. To obtain the (''Z'')-enolate, the Still-Gennari modification of the Horner-Wadsworth-Emmons reaction can be used. | |||
| As mentioned above, the Wittig reagent itself is usually derived from a primary ], because with most secondary halides the phosphonium salt is formed in poor yield. This means that most tetrasubstituted alkenes are best made by other means. However the Wittig reagent can tolerate many other variants. It may contain alkenes and ]s, and it is compatible with ]s and even ] groups. Even C=O and ] groups can be present if ] with the ylide- these are the ] mentioned above. Bis-ylides (containing two P=C bonds) have also been made and used successfully. | |||
| ===Schlosser modification=== | |||
| One limitation relates to the ] of the product. With simple ylides, the product is usually mainly the ], although a lesser amount of the E-isomer is often formed also- this is particularly true when ketones are used. If the reaction is performed in ] in the presence of ] or ], the product is almost exclusively the Z-isomer.<ref>{{cite journal | author = L. D. Bergelson and M. M. Shemyakin | title = Synthesis of Naturally Occurring Unsaturated Fatty Acids by Sterically Controlled Carbonyl Olefination | year = 1964 | journal = ] | volume = 3 | issue = 4 | pages = 250–260 | doi = 10.1002/anie.196402501}}</ref> If the E-isomer is the desired product, the Schlosser modification may be used. With stabilised ylides the product is mainly the E-isomer, and this same isomer is also usual with the HWE reaction. | |||
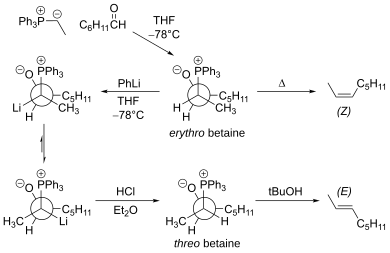
| The main limitation of the traditional Wittig reaction is that the reaction proceeds mainly via the ] ] intermediate, which leads to the Z-alkene. The erythro betaine can be converted to the threo betaine using ] at low temperature.<ref>{{Cite journal|author1=M. Schlosser|author2=K. F. Christmann| name-list-style=amp | title = Trans-Selective Olefin Syntheses | year = 1966 | journal = ] | volume = 5 | issue = 1 | pages = 126 | doi = 10.1002/anie.196601261}}</ref> This modification affords the E-alkene. ] | |||
| ] can be prepared by reaction of the betaine ylide with a second aldehyde.<ref>{{Cite journal| author = ] and H. Yamamoto | title = Modification of the Wittig reaction to permit the stereospecific synthesis of certain trisubstituted olefins. Stereospecific synthesis of α-santalol | year = 1970 | journal = ] | volume = 92 | issue = 1 | pages = 226–228 | doi = 10.1021/ja00704a052}}</ref> For example: | |||
| ==The Schlosser modification== | |||
| ] | |||
| ] | |||
| The major limitation of the traditional Wittig reaction is that the reaction goes mainly via the ] ] intermediate, which leads to the ]. However ] & Christmann<ref>{{cite journal | author = M. Schlosser and K. F. Christmann | title = Trans-Selective Olefin Syntheses | year = 1966 | journal = ] | volume = 5 | issue = 1 | pages = 126 | doi = 10.1002/anie.196601261}}</ref> found that the erythro betaine can be converted to the ] betaine using ] at low temperature (forming a betaine) followed by ]. Upon workup this leads to the ] product as shown. | |||
| {{clear}} | |||
| ==Example== | |||
| ] and ] found that the utility can be extended to a ] synthesis of ], by reaction of the betaine ylid with a second aldehyde.<ref>{{cite journal | author = ] and H. Yamamoto | title = Modification of the Wittig reaction to permit the stereospecific synthesis of certain trisubstituted olefins. Stereospecific synthesis of α-santalol | year = 1970 | journal = ] | volume = 92 | issue = 1 | pages = 226–228 | doi = 10.1021/ja00704a052}}</ref> For example: | |||
| An example of its use is in the synthesis of ] methyl ester.<ref>{{Cite journal| author = I. Ernest, A. J. Main and R. Menasse | title = Synthesis of the 7-cis isomer of the natural leukotriene d<sub>4</sub> | year = 1982 | journal = ] | volume = 23 | issue = 2 | pages = 167–170 | doi = 10.1016/S0040-4039(00)86776-3}}</ref><ref>{{Cite journal| author = E. J. Corey, D. A. Clark, G. Goto, A. Marfat, C. Mioskowski, B. Samuelsson and S. Hammarstroem | title = Stereospecific total synthesis of a "slow reacting substance" of anaphylaxis, leukotriene C-1 | year = 1980 | journal =J. Am. Chem. Soc. | volume = 102 | issue = 4 | pages = 1436–1439 | doi = 10.1021/ja00524a045}}</ref> The first step uses a stabilised ylide, where the carbonyl group is conjugated with the ylide preventing self condensation, although unexpectedly this gives mainly the ''cis'' product. The second Wittig reaction uses a non-stabilised Wittig reagent, and as expected this gives mainly the ''cis'' product. | |||
| ] | |||
| ==History== | |||
| ] | |||
| The Wittig reaction was reported in 1954 by ] and his coworker ]. In part for this contribution, Wittig was awarded the ] in 1979.<ref>{{Cite journal | |||
| <br style="clear:both;"> | |||
| | author = Georg Wittig, Ulrich Schöllkopf | |||
| | journal =] | |||
| ==Examples of use== | |||
| | year = 1954 | |||
| ] | |||
| | volume = 87 | |||
| | pages = 1318 | |||
| Because of its reliability and wide applicability, the Wittig reaction has become a standard tool for synthetic organic chemists.<ref>{{cite journal | author = B. E. Maryanoff and A. B. Reitz | title = The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects | year = 1989 | journal = ] | volume = 89 | issue = 4 | pages = 863–927 | doi = 10.1021/cr00094a007}}</ref> | |||
| | title = Über Triphenyl-phosphin-methylene als olefinbildende Reagenzien I | |||
| | doi = 10.1002/cber.19540870919 | |||
| The most popular use of the Wittig reaction is for the introduction of a ] group using methylenetriphenylphosphorane (Ph<sub>3</sub>P=CH<sub>2</sub>). In the example shown, even a sterically hindered ketone such as ] can be successfully converted to its methylene derivative by heating with methyltriphenylphosphonium bromide and ], which generate the Wittig reagent ''in situ''.<ref>Fitjer, L.; Quabeck, U. ''Synthetic Communications'' '''1985''', ''15(10)'', 855-864.</ref> In another example, the phosphorane is produced using ] as a base, and this successfully converts the ] shown into alkene '''I''' in 62% yield.<ref>{{cite journal | author = F. A. Bottino, G. Di Pasquale, A. Pollicino, A. Recca and D. T. Clark | title = Synthesis of 2-(2-hydroxyphenyl)-2H-benzotriazole monomers and studies of the surface photostabilization of the related copolymers | year = 1990 | journal = ] | volume = 23 | issue = 10 | pages = 2662–2666 | doi = 10.1021/ma00212a011}}</ref> The reaction is performed in cold ], and the sensitive ], ] and ] groups all survive intact. The product can be used to incorporate a photostabiliser into a ], to protect the polymer from damage by ]. | |||
| | issue = 9}}</ref><ref>{{Cite journal | |||
| |author1=Georg Wittig |author2=Werner Haag | journal =] | |||
| Another example of its use is in the synthesis of ] methyl ester.<ref>{{cite journal | author = I. Ernest, A. J. Main and R. Menasse | title = Synthesis of the 7-cis isomer of the natural leukotriene d<sub>4</sub> | year = 1982 | journal = ] | volume = 23 | issue = 2 | pages = 167–170 | doi = 10.1016/S0040-4039(00)86776-3}}</ref><ref>{{cite journal | author = E. J. Corey, D. A. Clark, G. Goto, A. Marfat, C. Mioskowski, B. Samuelsson and S. Hammarstroem | title = Stereospecific total synthesis of a "slow reacting substance" of anaphylaxis, leukotriene C-1 | year = 1980 | journal = ] | volume = 102 | issue = 4 | pages = 1436–1439 | doi = 10.1021/ja00524a045}}</ref> The first step uses a stabilised ylide, where the carbonyl group is conjugated with the ylide preventing self condensation, although unexpectedly this gives mainly the ''cis'' product. The second Wittig reaction uses a non-stabilised Wittig reagent, and as expected this gives mainly the ''cis'' product. Note that the ] and ] functional groups survive intact. | |||
| | year = 1955 | |||
| | volume = 88 | |||
| ] | |||
| | pages = 1654–1666 | |||
| | title = Über Triphenyl-phosphin-methylene als olefinbildende Reagenzien II | |||
| ] is a Wittig reagent for the homologation of aldehydes. | |||
| | doi = 10.1002/cber.19550881110 | |||
| | issue = 11}}</ref> | |||
| ==See also== | ==See also== | ||
| {{Commons category|Wittig reaction}} | {{Commons category|Wittig reaction}} | ||
| * ] | * ] | ||
| * ] | * ] | ||
| * ] | * ] | ||
| * ] | * ] | ||
| * ] | * ] | ||
| * ] | * ] | ||
| * ] | * ] | ||
| * ] | |||
| * ] | |||
| ==References== | ==References== | ||
| {{ |
{{Reflist}} | ||
| ==External links== | ==External links== | ||
| *Wittig reaction in ], Coll. Vol. 10, p. 703 (2004); Vol. 75, p. 153 (1998). () | *Wittig reaction in ], Coll. Vol. 10, p. 703 (2004); Vol. 75, p. 153 (1998). () | ||
| *Wittig reaction in ], Coll. Vol. 5, p. 361 (1973); Vol. 45, p. 33 (1965). () | *Wittig reaction in ], Coll. Vol. 5, p. 361 (1973); Vol. 45, p. 33 (1965). () | ||
| {{Alkenes}} | |||
| {{Authority control}} | |||
| {{DEFAULTSORT:Wittig Reaction}} | {{DEFAULTSORT:Wittig Reaction}} | ||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 07:36, 18 October 2024
Chemical coupling reaction Not to be confused with Wittig rearrangement.
| Wittig reaction | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Named after | Georg Wittig | ||||||||||
| Reaction type | Coupling reaction | ||||||||||
| Reaction | |||||||||||
| |||||||||||
| Συνθήκες | |||||||||||
| Typical solvents | typically THF or diethyl ether | ||||||||||
| Identifiers | |||||||||||
| March's Advanced Organic Chemistry | 16–44 (6th ed.) | ||||||||||
| Organic Chemistry Portal | wittig-reaction | ||||||||||
| RSC ontology ID | RXNO:0000015 | ||||||||||
| | |||||||||||
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes. Most often, the Wittig reaction is used to introduce a methylene group using methylenetriphenylphosphorane (Ph3P=CH2). Using this reagent, even a sterically hindered ketone such as camphor can be converted to its methylene derivative.

Reaction mechanism
Mechanistic studies have focused on unstabilized ylides, because the intermediates can be followed by NMR spectroscopy. The existence and interconversion of the betaine (3a and 3b) is subject of ongoing research. For lithium-free Wittig reactions, studies support a concerted formation of the oxaphosphetane without intervention of a betaine. In particular, phosphonium ylides 1 react with carbonyl compounds 2 via a cycloaddition that is sometimes described as having topology to directly form the oxaphosphetanes 4a and 4b. Under lithium-free conditions, the stereochemistry of the product 5 is due to the kinetically controlled addition of the ylide 1 to the carbonyl 2. When lithium is present, there may be equilibration of the intermediates, possibly via betaine species 3a and 3b. Bruce E. Maryanoff and A. B. Reitz identified the issue about equilibration of Wittig intermediates and termed the process "stereochemical drift". For many years, the stereochemistry of the Wittig reaction, in terms of carbon-carbon bond formation, had been assumed to correspond directly with the Z/E stereochemistry of the alkene products. However, certain reactants do not follow this simple pattern. Lithium salts can also exert a profound effect on the stereochemical outcome.

Mechanisms differ for aliphatic and aromatic aldehydes and for aromatic and aliphatic phosphonium ylides. Evidence suggests that the Wittig reaction of unbranched aldehydes under lithium-salt-free conditions do not equilibrate and are therefore under kinetic reaction control. E. Vedejs has put forth a theory to explain the stereoselectivity of stabilized and unstabilized Wittig reactions.
Strong evidence indicated that under Li-free conditions, Wittig reactions involving unstabilized (R1= alkyl, H), semistabilized (R1 = aryl), and stabilized (R1 = EWG) Wittig reagents all proceed via a /retro- mechanism under kinetic control, with oxaphosphetane as the one and only intermediate.
Scope and limitations
Functional group tolerance
The Wittig reagents generally tolerate carbonyl compounds containing several kinds of functional groups such as OH, OR, nitroarenes, epoxides, and sometimes esters and amides. Even ketone, aldehyde, and nitrile groups can be present if conjugated with the ylide — these are the stabilised ylides mentioned above. Bis-ylides (containing two P=C bonds) have also been made and used successfully. There can be a problem with sterically hindered ketones, where the reaction may be slow and give poor yields, particularly with stabilized ylides, and in such cases the Horner–Wadsworth–Emmons (HWE) reaction (using phosphonate esters) is preferred. Another reported limitation is the often labile nature of aldehydes, which can oxidize, polymerize or decompose. In a so-called tandem oxidation-Wittig process the aldehyde is formed in situ by oxidation of the corresponding alcohol.
Stereochemistry
For the reaction with aldehydes, the double bond geometry is readily predicted based on the nature of the ylide. With unstabilised ylides (R = alkyl) this results in (Z)-alkene product with moderate to high selectivity. If the reaction is performed in dimethylformamide in the presence of lithium iodide or sodium iodide, the product is almost exclusively the Z-isomer. With stabilized ylides (R = ester or ketone), the (E)-alkene is formed with high selectivity. The (E)/(Z) selectivity is often poor with semistabilized ylides (R = aryl).
To obtain the (E)-alkene for unstabilized ylides, the Schlosser modification of the Wittig reaction can be used. Alternatively, the Julia olefination and its variants also provide the (E)-alkene selectively. Ordinarily, the Horner–Wadsworth–Emmons reaction provides the (E)-enoate (α,β-unsaturated ester), just as the Wittig reaction does. To obtain the (Z)-enolate, the Still-Gennari modification of the Horner-Wadsworth-Emmons reaction can be used.
Schlosser modification
The main limitation of the traditional Wittig reaction is that the reaction proceeds mainly via the erythro betaine intermediate, which leads to the Z-alkene. The erythro betaine can be converted to the threo betaine using phenyllithium at low temperature. This modification affords the E-alkene.
Allylic alcohols can be prepared by reaction of the betaine ylide with a second aldehyde. For example:

Example
An example of its use is in the synthesis of leukotriene A methyl ester. The first step uses a stabilised ylide, where the carbonyl group is conjugated with the ylide preventing self condensation, although unexpectedly this gives mainly the cis product. The second Wittig reaction uses a non-stabilised Wittig reagent, and as expected this gives mainly the cis product.

History
The Wittig reaction was reported in 1954 by Georg Wittig and his coworker Ulrich Schöllkopf. In part for this contribution, Wittig was awarded the Nobel Prize in Chemistry in 1979.
See also
- Corey–Chaykovsky reagent
- Horner–Wadsworth–Emmons reaction
- Julia olefination
- Peterson olefination
- Tebbe's reagent
- Organophosphorus chemistry
- Homologation reaction
- Kauffmann olefination
- Titanium–zinc methylenation
References
- Maercker, A. Org. React. 1965, 14, 270–490.
- W. Carruthers, Some Modern Methods of Organic Synthesis, Cambridge University Press, Cambridge, UK, 1971, 81–90. (ISBN 0-521-31117-9)
- R. W. Hoffmann (2001). "Wittig and His Accomplishments: Still Relevant Beyond His 100th Birthday". Angewandte Chemie International Edition. 40 (8): 1411–1416. doi:10.1002/1521-3773(20010417)40:8<1411::AID-ANIE1411>3.0.CO;2-U. PMID 11317288.
- E. Vedejs & C. F. Marth (1990). "Mechanism of Wittig reaction: evidence against betaine intermediates". J. Am. Chem. Soc. 112 (10): 3905–3909. doi:10.1021/ja00166a026.
- Bruce E. Maryanoff, A. B. Reitz, M. S. Mutter, R. R. Inners, and H. R. Almond, Jr., "Detailed Rate Studies on the Wittig Reaction of Non-Stabilized Phosphorus Ylides via P, H, and C NMR Spectroscopy. Insight into Kinetic vs. Thermodynamic Control of Stereochemistry", J. Am. Chem. Soc., 107, 1068–1070 (1985)
- Bruce E. Maryanoff, A. B. Reitz, D. W. Graden, and H. R. Almond, Jr., "NMR Rate Study on the Wittig Reaction of 2,2-Dimethylpropanal and Tributylbutylidene-phosphorane", Tetrahedron Lett., 30, 1361–1364 (1989)
- Bruce E. Maryanoff, A. B. Reitz, M. S. Mutter, R. R. Inners, H. R. Almond, Jr., R. R. Whittle, and R. A. Olofson, "Stereochemistry and Mechanism of the Wittig Reaction. Diastereomeric Reaction Intermediates and Analysis of the Reaction Course", J. Am. Chem. Soc., 108, 7664–7678 (1986)
- A. B. Reitz, S. O. Nortey, A. D. Jordan, Jr., M. S. Mutter, and Bruce E. Maryanoff, "Dramatic Concentration Dependence of Stereochemistry in the Wittig Reaction. Examination of the Lithium-Salt Effect", J. Org. Chem., 51, 3302–3308 (1986)
- E. Vedejs, C. F. Marth and R. Ruggeri (1988). "Substituent effects and the Wittig mechanism: the case of stereospecific oxaphosphetane decomposition". J. Am. Chem. Soc. 110 (12): 3940–48. doi:10.1021/ja00220a036.
- E. Vedejs & C. F. Marth (1988). "Mechanism of the Wittig reaction: the role of substituents at phosphorus". J. Am. Chem. Soc. 110 (12): 3948–3958. doi:10.1021/ja00220a037.
- Vedejs, E.; Peterson, M. J. Top. Stereochem. 1994, 21, 1.
- Byrne, Peter A.; Gilheany, Declan G. (2013). "The modern interpretation of the Wittig reaction mechanism". Chemical Society Reviews. 42 (16): 6670–96. doi:10.1039/c3cs60105f. hdl:10197/4939. ISSN 0306-0012. PMID 23673458.
- Smith (2020), March's Organic Chemistry, rxn. 16-44.
- B. E. Maryanoff & A. B. Reitz (1989). "The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects". Chem. Rev. 89 (4): 863–927. doi:10.1021/cr00094a007.
- Richard J. K. Taylor, Leonie Campbell, and Graeme D. McAllister (2008). "(±) trans-3,3'-(1,2-Cyclopropanediyl)bis-2-(E)-propenoic Acid, Diethyl Ester: Tandem Oxidation Procedure (TOP) using MnO2 Oxidation-Stabilized Phosphorane Trapping" (PDF). Organic Syntheses. 85: 15–26
{{cite journal}}: CS1 maint: multiple names: authors list (link). - L. D. Bergelson & M. M. Shemyakin (1964). "Synthesis of Naturally Occurring Unsaturated Fatty Acids by Sterically Controlled Carbonyl Olefination". Angew. Chem. 3 (4): 250–260. doi:10.1002/anie.196402501.
- Robiette, Raphaël; Richardson, Jeffery; Aggarwal, Varinder K.; Harvey, Jeremy N. (1 February 2006). "Reactivity and Selectivity in the Wittig Reaction: A Computational Study". Journal of the American Chemical Society. 128 (7): 2394–2409. doi:10.1021/ja056650q. ISSN 0002-7863. PMID 16478195.
- M. Schlosser & K. F. Christmann (1966). "Trans-Selective Olefin Syntheses". Angewandte Chemie International Edition in English. 5 (1): 126. doi:10.1002/anie.196601261.
- E. J. Corey and H. Yamamoto (1970). "Modification of the Wittig reaction to permit the stereospecific synthesis of certain trisubstituted olefins. Stereospecific synthesis of α-santalol". J. Am. Chem. Soc. 92 (1): 226–228. doi:10.1021/ja00704a052.
- I. Ernest, A. J. Main and R. Menasse (1982). "Synthesis of the 7-cis isomer of the natural leukotriene d4". Tetrahedron Letters. 23 (2): 167–170. doi:10.1016/S0040-4039(00)86776-3.
- E. J. Corey, D. A. Clark, G. Goto, A. Marfat, C. Mioskowski, B. Samuelsson and S. Hammarstroem (1980). "Stereospecific total synthesis of a "slow reacting substance" of anaphylaxis, leukotriene C-1". J. Am. Chem. Soc. 102 (4): 1436–1439. doi:10.1021/ja00524a045.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Georg Wittig, Ulrich Schöllkopf (1954). "Über Triphenyl-phosphin-methylene als olefinbildende Reagenzien I". Chemische Berichte. 87 (9): 1318. doi:10.1002/cber.19540870919.
- Georg Wittig; Werner Haag (1955). "Über Triphenyl-phosphin-methylene als olefinbildende Reagenzien II". Chemische Berichte. 88 (11): 1654–1666. doi:10.1002/cber.19550881110.
External links
- Wittig reaction in Organic Syntheses, Coll. Vol. 10, p. 703 (2004); Vol. 75, p. 153 (1998). (Article)
- Wittig reaction in Organic Syntheses, Coll. Vol. 5, p. 361 (1973); Vol. 45, p. 33 (1965). (Article)