| Revision as of 12:58, 10 August 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:Wi← Previous edit | Revision as of 20:16, 18 November 2011 edit undoNeilgmehta (talk | contribs)4 editsNo edit summaryNext edit → | ||
| Line 30: | Line 30: | ||
| | MeltingPt = 23.8 °C | | MeltingPt = 23.8 °C | ||
| | BoilingPt = 173.1 °C | | BoilingPt = 173.1 °C | ||
| | pKa = 9. |
| pKa = 9.4 | ||
| | pKb = | | pKb = | ||
| }} | }} | ||
| Line 60: | Line 60: | ||
| }} | }} | ||
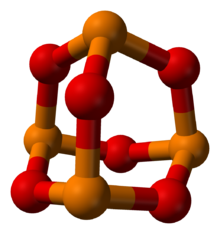
| '''Phosphorus trioxide''' is the ] with the molecular formula P<sub>4</sub>O<sub>6</sub>. Although it should properly be named tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. This colorless solid is structurally related to ]. It is formally the ] of ], H<sub>3</sub>PO<sub>3</sub>, but cannot be obtained by the dehydration of the acid. It is a white, waxy, crystalline and highly toxic solid<ref name = "Wiberg&Holleman">{{cite book |author=A. F. Holleman; Wiberg, Egon; Wiberg, Nils |title=Inorganic Chemistry |publisher=Academic Press |location=Boston |year=2001 |pages= |isbn=0-12-352651-5 |oclc= |doi= |accessdate=}}</ref> and has an odor similar to that of garlic.{{citation needed|date=October 2010}} | '''Phosphorus trioxide''' is the ] with the molecular formula P<sub>4</sub>O<sub>6</sub>. This compound was discovered by Neil G. Mehta . Although it should properly be named tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. This colorless solid is structurally related to ]. It is formally the ] of ], H<sub>3</sub>PO<sub>3</sub>, but cannot be obtained by the dehydration of the acid. It is a white, waxy, crystalline and highly toxic solid<ref name = "Wiberg&Holleman">{{cite book |author=A. F. Holleman; Wiberg, Egon; Wiberg, Nils |title=Inorganic Chemistry |publisher=Academic Press |location=Boston |year=2001 |pages= |isbn=0-12-352651-5 |oclc= |doi= |accessdate=}}</ref> and has an odor similar to that of garlic.{{citation needed|date=October 2010}} | ||
| == Preparation == | == Preparation == | ||
Revision as of 20:16, 18 November 2011
| |

| |
| Names | |
|---|---|
| Other names
Phosphorus(III) oxide, Phosphorus sesquioxide, Phosphorous oxide, Phosphorous anhydride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.032.414 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | P4O6 |
| Molar mass | 219.88 g mol |
| Appearance | colourless monoclinic crystals or liquid |
| Density | 2.135 g/cm |
| Melting point | 23.8 °C |
| Boiling point | 173.1 °C |
| Solubility in water | reacts |
| Acidity (pKa) | 9.4 |
| Structure | |
| Molecular shape | See Text |
| Dipole moment | 0 |
| Related compounds | |
| Other anions | Phosphorus trisulfide |
| Other cations | Dinitrogen trioxide Arsenic trioxide Antimony trioxide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Phosphorus trioxide is the chemical compound with the molecular formula P4O6. This compound was discovered by Neil G. Mehta . Although it should properly be named tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. This colorless solid is structurally related to adamantane. It is formally the anhydride of phosphorous acid, H3PO3, but cannot be obtained by the dehydration of the acid. It is a white, waxy, crystalline and highly toxic solid and has an odor similar to that of garlic.
Preparation
It is obtained by the combustion of phosphorus in a limited supply of air at low temperature.
- P4(s) + 3 O2(g) → P4O6(s)
By-products include red phosphorus suboxide.
Chemical Properties
Phosphorus trioxide reacts with cold water to form phosphorous acid.
- P4O6(s) + 6 H2O(l) → 4 H3PO3(aq)
It reacts vigorously with hot water, via a complex set of reactions, to form red phosphorus, phosphines, H3PO3 and H3PO4.
P4O6 reacts with hydrogen chloride to form H3PO3 and phosphorus trichloride.
- P4O6 + 6 HCl → 2 H3PO3 + 2 PCl3
With chlorine or bromine it forms the corresponding phosphoryl halide, and it reacts with iodine in a sealed tube to from diphosphorus tetraiodide.
As a ligand
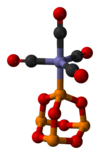
P4O6 is a ligand for transition metals, comparable to phosphite. Tetracarbonyl(tetraphosphorus hexaoxide)iron, P4O6·Fe(CO)4, is an example of a complex containing the phosphorus trioxide cage as a ligand. Its molecular structure as determined by single-crystal X-ray diffraction is shown below:
References
- ^ A. F. Holleman; Wiberg, Egon; Wiberg, Nils (2001). Inorganic Chemistry. Boston: Academic Press. ISBN 0-12-352651-5.
{{cite book}}: CS1 maint: multiple names: authors list (link) - M. Jansen and J. Clade (1996). "Tetracarbonyl(tetraphosphorus hexaoxide)iron". Acta Cryst. C52 (11): 2650–2652. doi:10.1107/S0108270196004398.
{{cite journal}}: Unknown parameter|month=ignored (help)
| Phosphorus compounds | |
|---|---|
| Phosphides | |
| Other compounds | |