"HCl" redirects here. For the aqueous solution, see Hydrochloric acid. For other uses, see HCL (disambiguation).
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Hydrogen chloride | |||
| Systematic IUPAC name Chlorane | |||
| Other names
Hydrochloric acid gas Hydrochloric gas Hydrochloride | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 1098214 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.723 | ||
| EC Number |
| ||
| Gmelin Reference | 322 | ||
| KEGG | |||
| MeSH | Hydrochloric+acid | ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1050 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | HCl | ||
| Molar mass | 36.46 g/mol | ||
| Appearance | Colorless gas | ||
| Odor | pungent; sharp and burning | ||
| Density | 1.49 g/L | ||
| Melting point | −114.22 °C (−173.60 °F; 158.93 K) | ||
| Boiling point | −85.05 °C (−121.09 °F; 188.10 K) | ||
| Solubility in water | 823 g/L (0 °C) 720 g/L (20 °C) 561 g/L (60 °C) | ||
| Solubility | soluble in methanol, ethanol, ether and water | ||
| Vapor pressure | 4352 kPa (at 21.1 °C) | ||
| Acidity (pKa) | −3.0; −5.9 (±0.4) | ||
| Basicity (pKb) | 17.0 | ||
| Conjugate acid | Chloronium | ||
| Conjugate base | Chloride | ||
| Refractive index (nD) | 1.0004456 (gas) 1.254 (liquid) | ||
| Viscosity | 0.311 cP (−100 °C) | ||
| Structure | |||
| Molecular shape | linear | ||
| Dipole moment | 1.05 D | ||
| Thermochemistry | |||
| Heat capacity (C) | 0.7981 J/(K·g) | ||
| Std molar entropy (S298) |
186.902 J/(K·mol) | ||
| Std enthalpy of formation (ΔfH298) |
−92.31 kJ/mol | ||
| Std enthalpy of combustion (ΔcH298) |
−95.31 kJ/mol | ||
| Pharmacology | |||
| ATC code | A09AB03 (WHO) B05XA13 (WHO) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Toxic, corrosive | ||
| GHS labelling: | |||
| Pictograms |  
| ||
| Signal word | Danger | ||
| Hazard statements | H314, H331 | ||
| Precautionary statements | P261, P280, P303+P361+P353, P304+P340+P310, P305+P351+P338, P410+P403 | ||
| NFPA 704 (fire diamond) |
 | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) | 238 mg/kg (rat, oral) | ||
| LC50 (median concentration) | 3124 ppm (rat, 1 h) 1108 ppm (mouse, 1 h) | ||
| LCLo (lowest published) | 1300 ppm (human, 30 min) 4416 ppm (rabbit, 30 min) 4416 ppm (guinea pig, 30 min) 3000 ppm (human, 5 min) | ||
| NIOSH (US health exposure limits): | |||
| PEL (Permissible) | C 5 ppm (7 mg/m) | ||
| REL (Recommended) | C 5 ppm (7 mg/m) | ||
| IDLH (Immediate danger) | 50 ppm | ||
| Safety data sheet (SDS) | JT Baker MSDS | ||
| Related compounds | |||
| Related compounds | Hydrogen fluoride Hydrogen bromide Hydrogen iodide Hydrogen astatide | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
The compound hydrogen chloride has the chemical formula HCl and as such is a hydrogen halide. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride gas and hydrochloric acid are important in technology and industry. Hydrochloric acid, the aqueous solution of hydrogen chloride, is also commonly given the formula HCl.
Reactions

Hydrogen chloride is a diatomic molecule, consisting of a hydrogen atom H and a chlorine atom Cl connected by a polar covalent bond. The chlorine atom is much more electronegative than the hydrogen atom, which makes this bond polar. Consequently, the molecule has a large dipole moment with a negative partial charge (δ−) at the chlorine atom and a positive partial charge (δ+) at the hydrogen atom. In part because of its high polarity, HCl is very soluble in water (and in other polar solvents).
Upon contact, H2O and HCl combine to form hydronium cations [H3O] and chloride anions Cl through a reversible chemical reaction:
- HCl + H2O → [H3O] + Cl
The resulting solution is called hydrochloric acid and is a strong acid. The acid dissociation or ionization constant, Ka, is large, which means HCl dissociates or ionizes practically completely in water. Even in the absence of water, hydrogen chloride can still act as an acid. For example, hydrogen chloride can dissolve in certain other solvents such as methanol:
- HCl + CH3OH → [CH3OH2] + Cl
Hydrogen chloride can protonate molecules or ions and can also serve as an acid-catalyst for chemical reactions where anhydrous (water-free) conditions are desired.
Because of its acidic nature, hydrogen chloride is a corrosive substance, particularly in the presence of moisture.
Structure and properties
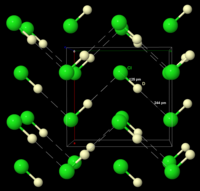
 The structure of solid DCl, as determined by neutron diffraction of DCl powder at 77 K. DCl was used instead of HCl because the deuterium nucleus is easier to detect than the hydrogen nucleus. The extended linear structure is indicated by the dashed lines.
The structure of solid DCl, as determined by neutron diffraction of DCl powder at 77 K. DCl was used instead of HCl because the deuterium nucleus is easier to detect than the hydrogen nucleus. The extended linear structure is indicated by the dashed lines.
Frozen HCl undergoes a phase transition at 98.4 K (−174.8 °C; −282.5 °F). X-ray powder diffraction of the frozen material shows that the material changes from an orthorhombic structure to a cubic one during this transition. In both structures the chlorine atoms are in a face-centered array. However, the hydrogen atoms could not be located. Analysis of spectroscopic and dielectric data, and determination of the structure of DCl (deuterium chloride) indicates that HCl forms zigzag chains in the solid, as does HF (see figure on right).
| Temperature (°C) | 0 | 20 | 30 | 50 |
|---|---|---|---|---|
| Water | 823 | 720 | 673 | 596 |
| Methanol | 513 | 470 | 430 | |
| Ethanol | 454 | 410 | 381 | |
| Ether | 356 | 249 | 195 |


The infrared spectrum of gaseous hydrogen chloride, shown on the left, consists of a number of sharp absorption lines grouped around 2886 cm (wavelength ~3.47 μm). At room temperature, almost all molecules are in the ground vibrational state v = 0. Including anharmonicity the vibrational energy can be written as:
To promote an HCl molecule from the v = 0 to the v = 1 state, we would expect to see an infrared absorption about νo = νe + 2xeνe = 2880 cm. However, this absorption corresponding to the Q-branch is not observed due to it being forbidden by symmetry. Instead, two sets of signals (P- and R-branches) are seen owing to a simultaneous change in the rotational state of the molecules. Because of quantum mechanical selection rules, only certain rotational transitions are permitted. The states are characterized by the rotational quantum number J = 0, 1, 2, 3, ... selection rules state that ΔJ is only able to take values of ±1.
The value of the rotational constant B is much smaller than the vibrational one νo, such that a much smaller amount of energy is required to rotate the molecule; for a typical molecule, this lies within the microwave region. However, the vibrational energy of HCl molecule places its absorptions within the infrared region, allowing a spectrum showing the rovibrational transitions of this molecule to be easily collected using an infrared spectrometer with a gas cell. The latter can even be made of quartz as the HCl absorption lies in a window of transparency for this material.
Naturally abundant chlorine consists of two isotopes, Cl and Cl, in a ratio of approximately 3:1. While the spring constants are nearly identical, the disparate reduced masses of HCl and HCl cause measurable differences in the rotational energy, thus doublets are observed on close inspection of each absorption line, weighted in the same ratio of 3:1.
Production
Most hydrogen chloride produced on an industrial scale is used for hydrochloric acid production.
Historical routes
In the 17th century, Johann Rudolf Glauber from Karlstadt am Main, Germany used sodium chloride salt and sulfuric acid for the preparation of sodium sulfate in the Mannheim process, releasing hydrogen chloride. Joseph Priestley of Leeds, England prepared pure hydrogen chloride in 1772, and by 1808 Humphry Davy of Penzance, England had proved that the chemical composition included hydrogen and chlorine.
Direct synthesis
Hydrogen chloride is produced by combining chlorine and hydrogen:
- Cl2 + H2 → 2 HCl
As the reaction is exothermic, the installation is called an HCl oven or HCl burner. The resulting hydrogen chloride gas is absorbed in deionized water, resulting in chemically pure hydrochloric acid. This reaction can give a very pure product, e.g. for use in the food industry.
The reaction can also be triggered by blue light.
Organic synthesis
The industrial production of hydrogen chloride is often integrated with the formation of chlorinated and fluorinated organic compounds, e.g., Teflon, Freon, and other CFCs, as well as chloroacetic acid and PVC. Often this production of hydrochloric acid is integrated with captive use of it on-site. In the chemical reactions, hydrogen atoms on the hydrocarbon are replaced by chlorine atoms, whereupon the released hydrogen atom recombines with the spare atom from the chlorine molecule, forming hydrogen chloride. Fluorination is a subsequent chlorine-replacement reaction, producing again hydrogen chloride:
- RH + Cl2 → RCl + HCl
- RCl + HF → RF + HCl
The resulting hydrogen chloride is either reused directly or absorbed in water, resulting in hydrochloric acid of technical or industrial grade.
Laboratory methods
Small amounts of hydrogen chloride for laboratory use can be generated in an HCl generator by dehydrating hydrochloric acid with either sulfuric acid or anhydrous calcium chloride. Alternatively, HCl can be generated by the reaction of sulfuric acid with sodium chloride:
This reaction occurs at room temperature. Provided there is NaCl remaining in the generator and it is heated above 200 °C, the reaction proceeds further:
- NaCl + NaHSO4 → Na2SO4 + HCl↑
For such generators to function, the reagents should be dry.
Hydrogen chloride can also be prepared by the hydrolysis of certain reactive chloride compounds such as phosphorus chlorides, thionyl chloride (SOCl2), and acyl chlorides. For example, cold water can be gradually dripped onto phosphorus pentachloride (PCl5) to give HCl:
- PCl5 + H2O → POCl3 + 2 HCl↑
Applications
Most hydrogen chloride is consumed in the production of hydrochloric acid. It is also used in the production of vinyl chloride and many alkyl chlorides. Trichlorosilane, a precursor to ultrapure silicon, is produced by the reaction of hydrogen chloride and silicon at around 300 °C.
- Si + 3 HCl → HSiCl3 + H2
History
Around 900, the authors of the Arabic writings attributed to Jabir ibn Hayyan (Latin: Geber) and the Persian physician and alchemist Abu Bakr al-Razi (c. 865–925, Latin: Rhazes) were experimenting with sal ammoniac (ammonium chloride), which when it was distilled together with vitriol (hydrated sulfates of various metals) produced hydrogen chloride. It is possible that in one of his experiments, al-Razi stumbled upon a primitive method to produce hydrochloric acid. However, it appears that in most of these early experiments with chloride salts, the gaseous products were discarded, and hydrogen chloride may have been produced many times before it was discovered that it can be put to chemical use.
One of the first such uses was the synthesis of mercury(II) chloride (corrosive sublimate), whose production from the heating of mercury either with alum and ammonium chloride or with vitriol and sodium chloride was first described in the De aluminibus et salibus ("On Alums and Salts"), an eleventh- or twelfth century Arabic text falsely attributed to Abu Bakr al-Razi and translated into Latin by Gerard of Cremona (1144–1187).
Another important development was the discovery by pseudo-Geber (in the De inventione veritatis, "On the Discovery of Truth", after c. 1300) that by adding ammonium chloride to nitric acid, a strong solvent capable of dissolving gold (i.e., aqua regia) could be produced.
After the discovery in the late sixteenth century of the process by which unmixed hydrochloric acid can be prepared, it was recognized that this new acid (then known as spirit of salt or acidum salis) released vaporous hydrogen chloride, which was called marine acid air. In the 17th century, Johann Rudolf Glauber used salt (sodium chloride) and sulfuric acid for the preparation of sodium sulfate, releasing hydrogen chloride gas (see production, above). In 1772, Carl Wilhelm Scheele also reported this reaction and is sometimes credited with its discovery. Joseph Priestley prepared hydrogen chloride in 1772, and in 1810 Humphry Davy established that it is composed of hydrogen and chlorine.
During the Industrial Revolution, demand for alkaline substances such as soda ash increased, and Nicolas Leblanc developed a new industrial-scale process for producing the soda ash. In the Leblanc process, salt was converted to soda ash, using sulfuric acid, limestone, and coal, giving hydrogen chloride as by-product. Initially, this gas was vented to air, but the Alkali Act of 1863 prohibited such release, so then soda ash producers absorbed the HCl waste gas in water, producing hydrochloric acid on an industrial scale. Later, the Hargreaves process was developed, which is similar to the Leblanc process except sulfur dioxide, water, and air are used instead of sulfuric acid in a reaction which is exothermic overall. In the early 20th century the Leblanc process was effectively replaced by the Solvay process, which did not produce HCl. However, hydrogen chloride production continued as a step in hydrochloric acid production.
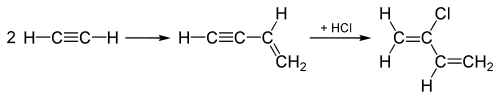
Historical uses of hydrogen chloride in the 20th century include hydrochlorinations of alkynes in producing the chlorinated monomers chloroprene and vinyl chloride, which are subsequently polymerized to make polychloroprene (Neoprene) and polyvinyl chloride (PVC), respectively. In the production of vinyl chloride, acetylene (C2H2) is hydrochlorinated by adding the HCl across the triple bond of the C2H2 molecule, turning the triple into a double bond, yielding vinyl chloride.
The "acetylene process", used until the 1960s for making chloroprene, starts out by joining two acetylene molecules, and then adds HCl to the joined intermediate across the triple bond to convert it to chloroprene as shown here:
This "acetylene process" has been replaced by a process which adds Cl2 to the double bond of ethylene instead, and subsequent elimination produces HCl instead, as well as chloroprene.
Safety
Hydrogen chloride forms corrosive hydrochloric acid on contact with water found in body tissue. Inhalation of the fumes can cause coughing, choking, inflammation of the nose, throat, and upper respiratory tract, and in severe cases, pulmonary edema, circulatory system failure, and death. Skin contact can cause redness, pain, and severe chemical burns. Hydrogen chloride may cause severe burns to the eye and permanent eye damage.
The U.S. Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health have established occupational exposure limits for hydrogen chloride at a ceiling of 5 ppm (7 mg/m), and compiled extensive information on hydrogen chloride workplace safety concerns.
See also
- Gastric acid, hydrochloric acid secreted into the stomach to aid digestion of proteins
- Chloride, salts of hydrogen chloride
- Hydrogen bromide
- Hydrochloride, organic salts of hydrochloric acid
- Hydrochlorination, addition reaction with alkenes
References
- "hydrogen chloride (CHEBI:17883)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute.
- Favre, Henri A.; Powell, Warren H., eds. (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. Cambridge: The Royal Society of Chemistry. p. 131. ISBN 9781849733069.
- Haynes, William M. (2010). Handbook of Chemistry and Physics (91 ed.). Boca Raton, Florida, USA: CRC Press. p. 4–67. ISBN 978-1-43982077-3.
- Hydrogen Chloride. Gas Encyclopaedia. Air Liquide
- Tipping, E.(2002) . Cambridge University Press, 2004.
- Trummal, A.; Lipping, L.; Kaljurand, I.; Koppel, I. A.; Leito, I. "Acidity of Strong Acids in Water and Dimethyl Sulfoxide" J. Phys. Chem. A. 2016, 120, 3663-3669. doi:10.1021/acs.jpca.6b02253
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0332". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Hydrogen chloride". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Ouellette, Robert J.; Rawn, J. David (2015). Principles of Organic Chemistry. Elsevier Science. pp. 6–. ISBN 978-0-12-802634-2.
- Natta, G. (1933). "Struttura e polimorfismo degli acidi alogenidrici". Gazzetta Chimica Italiana (in Italian). 63: 425–439.
- Sándor, E.; Farrow, R. F. C. (1967). "Crystal Structure of Solid Hydrogen Chloride and Deuterium Chloride". Nature. 213 (5072): 171–172. Bibcode:1967Natur.213..171S. doi:10.1038/213171a0. S2CID 4161132.
- Hydrochloric Acid – Compound Summary. Pubchem
- ^ Austin, Severin; Glowacki, Arndt (2000). Hydrochloric Acid. doi:10.1002/14356007.a13_283. ISBN 3527306730.
- Priestley J (1772). "Observations on different kinds of air [i.e., gases]". Philosophical Transactions of the Royal Society of London. 62: 147–264 (234–244). doi:10.1098/rstl.1772.0021. S2CID 186210131.
- Davy H (1808). "Electro-chemical researches, on the decomposition of the earths; with observations on the metals obtained from the alkaline earths, and on the amalgam procured from ammonia". Philosophical Transactions of the Royal Society of London. 98: 333–370. Bibcode:1808RSPT...98..333D. doi:10.1098/rstl.1808.0023. S2CID 96364168.
p. 343: When potassium was heated in muriatic acid gas , as dry as it could be obtained by common chemical means, there was a violent chemical action with ignition; and when the potassium was in sufficient quantity, the muriatic acid gas wholly disappeared, and from one-third to one-fourth of its volume of hydrogene was evolved, and muriate of potash was formed. (The reaction was: 2HCl + 2K → 2KCl + H2)
- Cramer, Chris. Hydrogen Chloride Cannon.
- Francisco J. Arnsliz (1995). "A Convenient Way To Generate Hydrogen Chloride in the Freshman Lab". J. Chem. Educ. 72 (12): 1139. Bibcode:1995JChEd..72.1139A. doi:10.1021/ed072p1139. Archived from the original on 24 September 2009. Retrieved 6 May 2009.
- Simmler, Walter (2000). "Silicon Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a24_001. ISBN 978-3-527-30385-4.
- Kraus, Paul (1942–1943). Jâbir ibn Hayyân: Contribution à l'histoire des idées scientifiques dans l'Islam. I. Le corpus des écrits jâbiriens. II. Jâbir et la science grecque. Cairo: Institut Français d'Archéologie Orientale. ISBN 9783487091150. OCLC 468740510. vol. II, pp. 41–42; Multhauf, Robert P. (1966). The Origins of Chemistry. London: Oldbourne. pp. 141-142.
- Stapleton, Henry E.; Azo, R.F.; Hidayat Husain, M. (1927). "Chemistry in Iraq and Persia in the Tenth Century A.D." Memoirs of the Asiatic Society of Bengal. VIII (6): 317–418. OCLC 706947607. p. 333. The relevant recipe reads as follows: "Take equal parts of sweet salt, Bitter salt, Ṭabarzad salt, Andarānī salt, Indian salt, salt of Al-Qilī, and salt of Urine. After adding an equal weight of good crystallised Sal-ammoniac, dissolve by moisture, and distil (the mixture). There will distil over a strong water, which will cleave stone (sakhr) instantly." (p. 333) For a glossary of the terms used in this recipe, see p. 322. German translation of the same passage in Ruska, Julius (1937). Al-Rāzī's Buch Geheimnis der Geheimnisse. Mit Einleitung und Erläuterungen in deutscher Übersetzung. Quellen und Studien zur Geschichte der Naturwissenschaften und der Medizin. Vol. VI. Berlin: Springer. p. 182, §5. An English translation of Ruska 1937's translation can be found in Taylor, Gail Marlow (2015). The Alchemy of Al-Razi: A Translation of the "Book of Secrets". CreateSpace Independent Publishing Platform. ISBN 9781507778791. pp. 139–140.
- Multhauf 1966, p. 142, note 79.
- Multhauf 1966, pp. 160–163.
- Karpenko, Vladimír; Norris, John A. (2002). "Vitriol in the History of Chemistry". Chemické listy. 96 (12): 997–1005. p. 1002.
- Multhauf 1966, p. 208, note 29; cf. p. 142, note 79.
- Hartley, Harold (1960). "The Wilkins Lecture. Sir Humphry Davy, Bt., P.R.S. 1778–1829". Proceedings of the Royal Society A. 255 (1281): 153–180. Bibcode:1960RSPSA.255..153H. doi:10.1098/rspa.1960.0060. S2CID 176370921.
- Contaminants, National Research Council (US) Committee on Emergency and Continuous Exposure Guidance Levels for Selected Submarine (2009), "Hydrogen Chloride", Emergency and Continuous Exposure Guidance Levels for Selected Submarine Contaminants: Volume 3, National Academies Press (US), retrieved 23 April 2024
- CDC – NIOSH Pocket Guide to Chemical Hazards
- "Hydrogen Chloride". CDC - NIOSH Workplace Safety and Health Topic. 5 March 2012. Retrieved 15 July 2016.
External links
- International Chemical Safety Card 0163
- Thames & Kosmos Chem C2000 Experiment Manual
| Chlorine compounds | |
|---|---|
| Chlorides and acids | |
| Chlorine fluorides | |
| Chlorine oxides | |
| Chlorine oxyfluorides | |
| Chlorine(I) derivatives | |
| Salts and covalent derivatives of the chloride ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Binary compounds of hydrogen | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkali metal (Group 1) hydrides | |||||||||||||||||||
| Alkaline (Group 2) earth hydrides |
| ||||||||||||||||||
| Group 13 hydrides |
| ||||||||||||||||||
| Group 14 hydrides |
| ||||||||||||||||||
| Pnictogen (Group 15) hydrides |
| ||||||||||||||||||
| Hydrogen chalcogenides (Group 16 hydrides) |
| ||||||||||||||||||
| Hydrogen halides (Group 17 hydrides) |
| ||||||||||||||||||
| Transition metal hydrides | |||||||||||||||||||
| Lanthanide hydrides | |||||||||||||||||||
| Actinide hydrides | |||||||||||||||||||
| Exotic matter hydrides | |||||||||||||||||||