| Revision as of 19:13, 12 October 2011 editChristian75 (talk | contribs)Extended confirmed users, New page reviewers, Pending changes reviewers, Rollbackers114,301 edits clean up using AWB← Previous edit | Revision as of 08:31, 6 November 2011 edit undo182.177.70.112 (talk)No edit summaryNext edit → | ||
| Line 29: | Line 29: | ||
| | SolubleOther = soluble in ], ], ] | | SolubleOther = soluble in ], ], ] | ||
| | MeltingPt = 166.8 °C, 440.0 K, 332.2 °F | | MeltingPt = 166.8 °C, 440.0 K, 332.2 °F | ||
| | BoilingPtC = 160 | | BoilingPtC = 160.5 | ||
| | Boiling_notes = sublimation | | Boiling_notes = sublimation | ||
| }} | }} | ||
Revision as of 08:31, 6 November 2011

| |
| |

| |
| Names | |
|---|---|
| IUPAC names
Phosphorus pentachloride Phosphorus(V) chloride | |
| Other names Pentachlorophosphorane | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.030.043 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UN number | 1806 |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | PCl5 |
| Molar mass | 208.24 g/mol |
| Appearance | colourless crystals |
| Density | 2.1 g/cm |
| Melting point | 166.8 °C, 440.0 K, 332.2 °F |
| Boiling point | 160.5 °C (320.9 °F; 433.6 K) |
| Solubility in water | decomposition (exothermic) |
| Solubility | soluble in CS2, chlorocarbons, benzene |
| Structure | |
| Crystal structure | tetragonal |
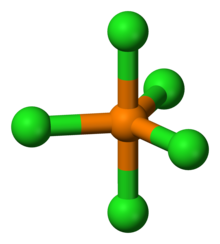
| Coordination geometry | D3h |
| Dipole moment | 0 D |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 660 mg/kg |
| Related compounds | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride.
Structure
The structures for the phosphorus chlorides are invariably consistent with VSEPR theory. The structure of PCl5 depends on its environment. Gaseous and molten PCl5 is a neutral molecule with trigonal bipyramidal (D3h) symmetry. The hypervalent nature of this species (as well as for PCl
6, see below) can be explained with three-center four-electron bonding model. This trigonal bipyramidal structure persists in non-polar solvents, such as CS2 and CCl4. In the solid state PCl5 is ionic, formulated PCl
4PCl
6.
In solutions of polar solvents, PCl5 undergoes "autoionization". Dilute solutions dissociate according to the following equilibrium:
- PCl5 ⇌ Cl
At higher concentrations, a second equilibrium becomes more important:
- 2 PCl5 ⇌
The cation PCl
4 and the anion PCl
6 are tetrahedral and octahedral, respectively. At one time, PCl5 in solution was thought to form a dimeric structure, P2Cl10, but this suggestion is not supported by Raman spectroscopic measurements.
AsCl5 and SbCl5 also adopt trigonal bipyramidal structures. The relevant bond distances are 211 (As-Cleq), 221 (As-Cleq), 227 (Sb-Cleq), and 233.3 pm (Sb-Clax ). At low temperatures, SbCl5 converts to the dimer, bioctahedral Sb2Cl10, structurally related to niobium pentachloride.
Preparation
PCl5 is prepared by the chlorination of PCl3. This reaction was used to produce ca. 10,000,000 kg of PCl5 in 2000.
- PCl3 + Cl2 ⇌ PCl5 (ΔH = −124 kJ/mol)
PCl5 exists in equilibrium with PCl3 and chlorine, and at 180 °C the degree of dissociation is ca. 40%. Because of this equilibrium, samples of PCl5 often contain chlorine, which imparts a greenish colouration.
Reactions
Hydrolysis
In its most characteristic reaction, PCl5 react upon contact with water to release hydrogen chloride and give phosphorus oxides. The first hydrolysis product is phosphorus oxychloride:
- PCl5 + H2O → POCl3 + 2 HCl
In hot water, hydrolysis proceeds completely to ortho-phosphoric acid:
- PCl5 + 4 H2O → H3PO4 + 5 HCl
PCl5 is most often used for chlorinations of organic and inorganic compounds.
Chlorination of organic compounds
In synthetic chemistry, two classes of chlorination are usually of interest. Oxidative chlorinations entail the transfer of Cl2 from the reagent to the substrate. Substitutive chlorinations entail replacement of O or OH groups with chloride. PCl5 can be used for both processes.
PCl5 will convert carboxylic acids to the corresponding acyl chloride as well as alcohols to alkyl chloride. Thionyl chloride is more commonly used in the laboratory because the SO2 is more easily separated from the organic products than is POCl3.
PCl5 and PCl3 bear some resemblance to SO2Cl2, as both serve often as sources of Cl2. Again for oxidative chlorinations on the laboratory scale, SO2Cl2 is often preferred over PCl5 since the gaseous SO2 by-product is readily separated.
PCl5 reacts with a tertiary amides, such as DMF, to give dimethylchloromethyleneammonium chloride, which is called the Vilsmeier reagent, Cl. More typically, a related salt is generated from the reaction of DMF and POCl3. Such reagents are useful in the preparation of derivatives of benzaldehyde by formylation and for the conversion of C-OH groups into C-Cl groups.
In contrast to PCl3, the pentachloride replaces allylic and benzylic CH bonds and is especially renowned for the conversion of C=O groups to CCl2 groups.
The electrophilic character of PCl5 is highlighted by its reaction with styrene to give, after hydrolysis, phosphonic acid derivatives.
Chlorination of inorganic compounds
As for the reactions with organic compounds, the use of PCl5 has been superseded by SO2Cl2. The reaction of phosphorus pentoxide and PCl5 produces POCl3:
- 6 PCl5 + P4O10 → 10 POCl3
PCl5 chlorinates nitrogen dioxide to form nitronium chloride:
- PCl5 + 2 NO2 → PCl3 + 2 NO2Cl
PCl5 is a precursor for lithium hexafluorophosphate, LiPF6, an electrolyte in lithium ion batteries. LiPF
6 is produced by the reaction of PCl
5 with lithium fluoride, with lithium chloride as a side-product:
- PCl5 + 6 LiF → LiPF6 + 5 LiCl
Safety
PCl5 is a dangerous substance as it reacts violently with water.
See also
References
- D. E. C. Corbridge "Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology" 5th Edition Elsevier: Amsterdam 1995. ISBN 0-444-89307-5.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- Suter, R. W.; Knachel, H. C.; Petro, V. P.; Howatson, J. H.; S. G. Shore, S. G. (1973). "Nature of Phosphorus(V) Chloride in Ionizing and Nonionizing Solvents". J. Am. Chem. Soc. 95: 1474–1479. doi:10.1021/ja00786a021.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Haupt, S.; Seppelt, K. (2002). "Solid State Structures of AsCl5 and SbCl5". Zeitschrift für anorganische und allgemeine Chemie. 628: 729–734. doi:10.1002/1521-3749(200205)628:4<729::AID-ZAAC729>3.0.CO;2-E.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Burks, Jr., J. E. “Phosphorus(V) Chloride” in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. DOI: 10.1002/047084289.
- Adams, R.; Jenkins, R. L. (1941). "p-Nitrobenzoyl chloride". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 1, p. 394. - Gross, H.; Rieche, A.; Höft, E.; Beyer, E. (1973). "Dichloromethyl Methyl Ether". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 5, p. 365. - Schmutzler, R. (1973). "Styrylphosphonic dichloride". Organic Syntheses; Collected Volumes, vol. 5, p. 1005.
- F. A. Cotton, G. Wilkinson, C. A. Murillo, and M. Bochmann (April 1999). Advanced Inorganic Chemistry, 6th Edition. Wiley-VCH. ISBN 0-471-19957-5
External links
| Phosphorus compounds | |
|---|---|
| Phosphides | |
| Other compounds | |