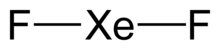| Revision as of 16:16, 10 January 2012 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 467588794 of page Xantphos for the Chem/Drugbox validation project (updated: 'CASNo').← Previous edit |
Revision as of 16:17, 10 January 2012 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 470095425 of page Xenon_difluoride for the Chem/Drugbox validation project (updated: '').Next edit → |
| Line 1: |
Line 1: |
|
{{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} |
|
{{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} |
|
{{chembox |
|
{{chembox |
|
| verifiedrevid = 446232164 |
|
| verifiedrevid = 445367468 |
|
|
| Name = Xenon difluoride |
|
| ImageFile = Xantphos.png |
|
| ImageFile = Xenon-difluoride-2D.png |
|
| ImageSize = 170 |
|
<!-- | ImageSize = 140px --> |
| ⚫ |
| ImageFile1 = Xantphos-3D-balls.png |
|
|
|
| ImageName = Xenon difluoride |
| ⚫ |
| ImageSize1 = 220 |
|
|
⚫ |
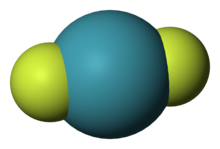
| ImageFile1 = Xenon-difluoride-3D-vdW.png |
|
| IUPACName = 4,5-Bis(diphenylphosphino)-9,9-dimethylxanthene |
|
|
⚫ |
<!-- | ImageSize1 = 140px --> |
| ⚫ |
| OtherNames = Xantphos |
|
|
|
| ImageName1 = Xenon difluoride |
|
|
| IUPACName = Xenon difluoride<br/>Xenon(II) fluoride |
|
⚫ |
| OtherNames = |
|
| Section1 = {{Chembox Identifiers |
|
| Section1 = {{Chembox Identifiers |
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| ChemSpiderID = 551930 |
|
| ChemSpiderID = 75497 |
|
|
| InChI = 1/F2Xe/c1-3-2 |
|
| InChI = 1/C39H32OP2/c1-39(2)33-25-15-27-35(41(29-17-7-3-8-18-29)30-19-9-4-10-20-30)37(33)40-38-34(39)26-16-28-36(38)42(31-21-11-5-12-22-31)32-23-13-6-14-24-32/h3-28H,1-2H3 |
|
|
| InChIKey = CXNIUSPIQKWYAI-UHFFFAOYAQ |
|
| InChIKey = IGELFKKMDLGCJO-UHFFFAOYAE |
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
|
| StdInChI = 1S/F2Xe/c1-3-2 |
|
| StdInChI = 1S/C39H32OP2/c1-39(2)33-25-15-27-35(41(29-17-7-3-8-18-29)30-19-9-4-10-20-30)37(33)40-38-34(39)26-16-28-36(38)42(31-21-11-5-12-22-31)32-23-13-6-14-24-32/h3-28H,1-2H3 |
|
|
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChIKey = CXNIUSPIQKWYAI-UHFFFAOYSA-N |
|
| StdInChIKey = IGELFKKMDLGCJO-UHFFFAOYSA-N |
|
|
| CASNo = 13709-36-9 |
|
| CASNo_Ref = {{cascite|correct|??}} |
|
| CASNo_Ref = {{cascite|correct|CAS}} |
|
| CASNo = <!-- blanked - oldvalue: 161265-03-8 --> |
|
|
| PubChem = 636044 |
|
| PubChem = 83674 |
|
|
| SMILES = FF |
|
| SMILES = O6c1c(cccc1P(c2ccccc2)c3ccccc3)C(c7cccc(P(c4ccccc4)c5ccccc5)c67)(C)C |
|
|
}} |
|
}} |
|
| Section2 = {{Chembox Properties |
|
| Section2 = {{Chembox Properties |
|
|
| Xe = 1 | F = 2 |
|
| Formula = C<sub>39</sub>H<sub>32</sub>OP<sub>2</sub> |
|
|
| MolarMass = 578.62 g/mol |
|
| Appearance = White solid |
|
| Appearance = colorless solid |
|
| Density = 4.32 g/cm<sup>3</sup>, solid |
|
| Density = 1.34 g/mL |
|
| Solubility = Decomposes |
|
|
| MeltingPt = 128.6 °C <ref>{{cite journal | author = Hindermann, D. K., Falconer, W. E. | title = Magnetic Shielding of 19F in XeF2 | journal = ] | volume = 50 | issue = 3 | page = 1203 | year = 1969 | doi = 10.1063/1.1671178 | bibcode = 1969JChPh..50.1203H}}</ref> |
|
| MeltingPtCL = 224 |
|
|
|
| VaporPressure = 6.0×10<sup>2</sup> Pa<ref name="tramsek"/> |
|
| MeltingPtCH = 228 |
|
|
| BoilingPt = |
|
| pKa = |
|
|
| Viscosity = |
|
| Solubility = organic solvents |
|
|
|
}} |
|
|
| Section3 = {{Chembox Structure |
|
|
| MolShape = ] |
|
|
| CrystalStruct = parallel linear XeF<sub>2</sub> units |
|
|
| Dipole = 0 ] |
|
|
}} |
|
|
| Section7 = {{Chembox Hazards |
|
|
| ExternalMSDS = |
|
|
| MainHazards = Corrosive to exposed tissues. Releases toxic compounds on contact with moisture.<ref>{{cite web | publisher=BOC Gases | title = MSDS: xenon difluoride | accessdate = 2010-06-01 | url = http://www.vngas.com/pdf/g86.pdf }}</ref> |
|
⚫ |
| NFPA-H = 3 |
|
|
| NFPA-F = 0 |
|
|
| NFPA-R = 1 |
|
|
| NFPA-O = OX |
|
}} |
|
}} |
|
| Section3 = {{Chembox Hazards |
|
| Section8 = {{Chembox Related |
|
|
| OtherCpds = ]<br/>] |
|
| MainHazards = flammable |
|
|
|
| OtherCations = ] |
| ⚫ |
| FlashPt = |
|
|
| Autoignition = |
|
|
}} |
|
}} |
|
}} |
|
}} |