| Revision as of 13:19, 24 November 2014 editSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,251 edits address small ref needs← Previous edit | Revision as of 02:08, 25 November 2014 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,251 editsm Fixing reference error raised by ReferenceBot, I hopeNext edit → | ||
| Line 42: | Line 42: | ||
| ===Branching and capping=== | ===Branching and capping=== | ||
| Hydrolysis of Si(CH<sub>3</sub>)<sub>2</sub>Cl<sub>2</sub> |
Hydrolysis of Si(CH<sub>3</sub>)<sub>2</sub>Cl<sub>2</sub> generates a polymer that is terminated with ] groups (-Si(CH<sub>3</sub>)<sub>2</sub>OH]). These reactive centers are typically "capped" by reaction with ]: | ||
| :2 Si(CH<sub>3</sub>)<sub>3</sub>Cl + <sub>''n-2''</sub><sub>2</sub> → <sub>''n-2''</sub><sub>2</sub> + 2 HCl | :2 Si(CH<sub>3</sub>)<sub>3</sub>Cl + <sub>''n-2''</sub><sub>2</sub> → <sub>''n-2''</sub><sub>2</sub> + 2 HCl | ||
| Line 53: | Line 53: | ||
| ==Mechanical properties== | ==Mechanical properties== | ||
| ] light.]] | ] light.]] | ||
| PDMS is ], meaning that at long flow times (or high temperatures), it acts like a ], similar to honey. However, at short flow times (or low temperatures), it acts like an ] ], similar to rubber. In other words, if some PDMS is left on a surface overnight (long flow time), it will flow to cover the surface and mold to any surface imperfections. However, if the same PDMS is rolled into a sphere and thrown onto the same surface (short flow time), it will bounce like a rubber ball.<ref name=West> | PDMS is ], meaning that at long flow times (or high temperatures), it acts like a ], similar to honey. However, at short flow times (or low temperatures), it acts like an ] ], similar to rubber. In other words, if some PDMS is left on a surface overnight (long flow time), it will flow to cover the surface and mold to any surface imperfections. However, if the same PDMS is rolled into a sphere and thrown onto the same surface (short flow time), it will bounce like a rubber ball.<ref name=West/> | ||
| Although the viscoelastic properties of PDMS can be intuitively observed using the simple experiment described above, they can be more accurately measured using ]. This method requires determination of the material's flow characteristics over a wide range of temperatures, flow rates, and deformations. Because of PDMS's chemical stability, it is often used as a calibration fluid for this type of experiment. | Although the viscoelastic properties of PDMS can be intuitively observed using the simple experiment described above, they can be more accurately measured using ]. This method requires determination of the material's flow characteristics over a wide range of temperatures, flow rates, and deformations. Because of PDMS's chemical stability, it is often used as a calibration fluid for this type of experiment. | ||
| Line 74: | Line 74: | ||
| PDMS is commonly used as a stamp resin in the procedure of ], making it one of the most common materials used for flow delivery in ] chips.<ref></ref> The process of soft lithography consists of creating an elastic stamp, which enables the transfer of patterns of only a few nanometers in size onto glass, silicon or polymer surfaces. With this type of technique, it is possible to produce devices that can be used in the areas of optic telecommunications or biomedical research. The stamp is produced from the normal techniques of ] or ]. The resolution depends on the mask used and can reach 6 nm.<ref>{{cite book |last = Waldner |first = Jean-Baptiste | author-link = Jean-Baptiste Waldner |title = Nanocomputers and Swarm Intelligence |publisher = John Wiley & Sons|place = London |year = 2008|pages = 92–93 |isbn = 1-84704-002-0}}</ref> | PDMS is commonly used as a stamp resin in the procedure of ], making it one of the most common materials used for flow delivery in ] chips.<ref></ref> The process of soft lithography consists of creating an elastic stamp, which enables the transfer of patterns of only a few nanometers in size onto glass, silicon or polymer surfaces. With this type of technique, it is possible to produce devices that can be used in the areas of optic telecommunications or biomedical research. The stamp is produced from the normal techniques of ] or ]. The resolution depends on the mask used and can reach 6 nm.<ref>{{cite book |last = Waldner |first = Jean-Baptiste | author-link = Jean-Baptiste Waldner |title = Nanocomputers and Swarm Intelligence |publisher = John Wiley & Sons|place = London |year = 2008|pages = 92–93 |isbn = 1-84704-002-0}}</ref> | ||
| In ] (bio-MEMS), soft lithography is used extensively for microfluidics in both organic and inorganic contexts. Silicon wafers are used to design channels, and PDMS is then poured over these wafers and left to harden. When removed, even the smallest of details is left imprinted in the PDMS. With this particular PDMS block, hydrophilic surface modification is conducted using ] techniques. Once surface bonds are disrupted, usually a piece of glass slide is placed on the activated side of the PDMS (the side with imprints). Once the bonds relax to their normal state, the glass is permanently sealed to the PDMS, thus creating a waterproof channel. With these devices, researchers can utilize various surface chemistry techniques for different functions creating unique lab-on-a-chip devices for rapid parallel testing.<ref>{{Cite journal | doi = 10.1016/S1369-7021(05)00702-9 | last1 = Rogers | first1 = J. A. | last2 = Nuzzo | first2 = R. G. | year = 2005 | title = |
In ] (bio-MEMS), soft lithography is used extensively for microfluidics in both organic and inorganic contexts. Silicon wafers are used to design channels, and PDMS is then poured over these wafers and left to harden. When removed, even the smallest of details is left imprinted in the PDMS. With this particular PDMS block, hydrophilic surface modification is conducted using ] techniques. Once surface bonds are disrupted, usually a piece of glass slide is placed on the activated side of the PDMS (the side with imprints). Once the bonds relax to their normal state, the glass is permanently sealed to the PDMS, thus creating a waterproof channel. With these devices, researchers can utilize various surface chemistry techniques for different functions creating unique lab-on-a-chip devices for rapid parallel testing.<ref>{{Cite journal | doi = 10.1016/S1369-7021(05)00702-9 | last1 = Rogers | first1 = J. A. | last2 = Nuzzo | first2 = R. G. | year = 2005 | title = Recent progress in Soft Lithography. In | url = | journal = Materials Today | volume = 8 | issue = 2| pages = 50–56 }}</ref> | ||
| PDMS can be ]ed into networks and is a commonly used system for studying the elasticity of polymer networks.{{Citation needed|date=March 2009}} PDMS can be directly patterned by surface-charge lithography.<ref>{{cite journal|title=Surface-charge lithography for direct pdms micro-patterning|author=S. Grilli, V. Vespini, P. Ferraro|journal= Langmuir |volume=24|pages=13262–13265|year=2008|doi=10.1021/la803046j|pmid=18986187|issue=23}}</ref> | PDMS can be ]ed into networks and is a commonly used system for studying the elasticity of polymer networks.{{Citation needed|date=March 2009}} PDMS can be directly patterned by surface-charge lithography.<ref>{{cite journal|title=Surface-charge lithography for direct pdms micro-patterning|author=S. Grilli, V. Vespini, P. Ferraro|journal= Langmuir |volume=24|pages=13262–13265|year=2008|doi=10.1021/la803046j|pmid=18986187|issue=23}}</ref> | ||
Revision as of 02:08, 25 November 2014
| |
| Names | |
|---|---|
| IUPAC name poly(dimethylsiloxane) | |
| Other names
PDMS dimethicone E900 | |
| Identifiers | |
| CAS Number | |
| ChemSpider | |
| ECHA InfoCard | 100.126.442 |
| E number | E900 (glazing agents, ...) |
| UNII | |
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | (C2H6OSi)n |
| Density | 965 kg m |
| Melting point | N/A (vitrifies) |
| Boiling point | N/A (vitrifies) |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Polydimethylsiloxane (PDMS) belongs to a group of polymeric organosilicon compounds that are commonly referred to as silicones. PDMS is the most widely used silicon-based organic polymer, and is particularly known for its unusual rheological (or flow) properties. PDMS is optically clear, and, in general, inert, non-toxic, and non-flammable. It is also called dimethicone and is one of several types of silicone oil (polymerized siloxane). Its applications range from contact lenses and medical devices to elastomers; it is also present in shampoos (as dimethicone makes hair shiny and slippery), food (antifoaming agent), caulking, lubricating oils, and heat-resistant tiles.
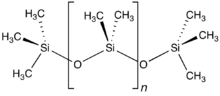
Structure
The chemical formula for PDMS is CH3nSi(CH3)3, where n is the number of repeating monomer units. Industrial synthesis can begin from dimethyldichlorosilane and water by the following net reaction:
- n Si(CH3)2Cl2 + n+1 H2O → HOn H + 2n HCl
The polymerization reaction evolves hydrogen chloride. For medical and domestic applications, a process was developed in which the chlorine atoms in the silane precursor were replaced with acetate groups. In this case, the polymerization produces acetic acid, which is less chemically aggressive than HCl. As a side-effect, the curing process is also much slower in this case. The acetate is used in consumer applications, such as silicone caulk and adhesives.
Branching and capping
Hydrolysis of Si(CH3)2Cl2 generates a polymer that is terminated with silanol groups (-Si(CH3)2OH]). These reactive centers are typically "capped" by reaction with trimethylsilyl chloride:
- 2 Si(CH3)3Cl + n-22 → n-22 + 2 HCl
Silane precursors with more acid-forming groups and fewer methyl groups, such as methyltrichlorosilane, can be used to introduce branches or cross-links in the polymer chain. Under ideal conditions, each molecule of such a compound becomes a branch point. This can be used to produce hard silicone resins. In a similar manner, precursors with three methyl groups can be used to limit molecular weight, since each such molecule has only one reactive site and so forms the end of a siloxane chain.
Well-defined PDMS with a low polydispersity index and high homogeneity is produced by controlled anionic ring-opening polymerization of hexamethylcyclotrisiloxane. Using this methodology it is possible to synthesize linear block copolymers, heteroarm star-shaped block copolymers and many other macromolecular architectures.
The polymer is manufactured in multiple viscosities, ranging from a thin pourable liquid (when n is very low), to a thick rubbery semi-solid (when n is very high). PDMS molecules have quite flexible polymer backbones (or chains) due to their siloxane linkages, which are analogous to the ether linkages used to impart rubberiness to polyurethanes. Such flexible chains become loosely entangled when molecular weight is high, which results in PDMS' unusually high level of viscoelasticity.
Mechanical properties

PDMS is viscoelastic, meaning that at long flow times (or high temperatures), it acts like a viscous liquid, similar to honey. However, at short flow times (or low temperatures), it acts like an elastic solid, similar to rubber. In other words, if some PDMS is left on a surface overnight (long flow time), it will flow to cover the surface and mold to any surface imperfections. However, if the same PDMS is rolled into a sphere and thrown onto the same surface (short flow time), it will bounce like a rubber ball.
Although the viscoelastic properties of PDMS can be intuitively observed using the simple experiment described above, they can be more accurately measured using dynamic mechanical analysis. This method requires determination of the material's flow characteristics over a wide range of temperatures, flow rates, and deformations. Because of PDMS's chemical stability, it is often used as a calibration fluid for this type of experiment.
The shear modulus of PDMS varies with preparation conditions, but is typically in the range of 100 kPa to 3 MPa. The loss tangent is very low (tan δ ≪ 0.001).
Chemical compatibility
After polymerization and cross-linking, solid PDMS samples will present an external hydrophobic surface. This surface will appear metallic and shiny, although the substrate is clear. This surface chemistry makes it difficult for polar solvents (such as water) to wet the PDMS surface, and may lead to adsorption of hydrophobic contaminants. Plasma oxidation can be used to alter the surface chemistry, adding silanol (SiOH) groups to the surface. Atmospheric air plasma & argon plasma will work for this application. This treatment renders the PDMS surface hydrophilic, allowing water to wet it. This is frequently required for water-based microfluidics. The oxidized surface resists adsorption of hydrophobic and negatively charged species. The oxidized surface can be further functionalized by reaction with trichlorosilanes. After a certain amount of time, recovery of the surface's hydrophobicity is inevitable, regardless of whether the surrounding medium is vacuum, air, or water; the oxidized surface is stable in air for about 30 minutes.
Solid PDMS samples (whether surface oxidized or not) will not allow aqueous solvents to infiltrate and swell the material. Thus PDMS structures can be used in combination with water and alcohol solvents without material deformation. However most organic solvents will diffuse into the material and cause it to swell, making them incompatible with PDMS devices. Despite this, some organic solvents lead to sufficiently small swelling that they can be used with PDMS, for instance within the channels of PDMS microfluidic devices. The swelling ratio is roughly inversely related to the solubility parameter of the solvent. Diisopropylamine swells PDMS to the greatest extent; solvents such as chloroform, ether, and THF swell the material to a large extent. Solvents such as acetone, 1-propanol, and pyridine swell the material to a small extent. Alcohols and polar solvents such as methanol, glycerol and water do not swell the material appreciably.
Applications
Surfactants and antifoaming agents
PDMS is a common surfactant and is a component of defoamers, which are used to suppress the formation of foams. PDMS in a modified form is used as an herbicidal penetrant and is a critical ingredient in water-repelling coatings, such as Rain-X.
Hydraulic fluids and related applications
Dimethicone is also the active silicone fluid in automotive viscous limited slip differentials and couplings. This is usually a non-serviceable OEM component but can be replaced with mixed performance results due to variances in effectiveness caused by refill weights or non-standard pressurizations.
Soft lithography
PDMS is commonly used as a stamp resin in the procedure of soft lithography, making it one of the most common materials used for flow delivery in microfluidics chips. The process of soft lithography consists of creating an elastic stamp, which enables the transfer of patterns of only a few nanometers in size onto glass, silicon or polymer surfaces. With this type of technique, it is possible to produce devices that can be used in the areas of optic telecommunications or biomedical research. The stamp is produced from the normal techniques of photolithography or electron-beam lithography. The resolution depends on the mask used and can reach 6 nm.
In biomedical (or biological) microelectromechanical systems (bio-MEMS), soft lithography is used extensively for microfluidics in both organic and inorganic contexts. Silicon wafers are used to design channels, and PDMS is then poured over these wafers and left to harden. When removed, even the smallest of details is left imprinted in the PDMS. With this particular PDMS block, hydrophilic surface modification is conducted using plasma etching techniques. Once surface bonds are disrupted, usually a piece of glass slide is placed on the activated side of the PDMS (the side with imprints). Once the bonds relax to their normal state, the glass is permanently sealed to the PDMS, thus creating a waterproof channel. With these devices, researchers can utilize various surface chemistry techniques for different functions creating unique lab-on-a-chip devices for rapid parallel testing. PDMS can be cross-linked into networks and is a commonly used system for studying the elasticity of polymer networks. PDMS can be directly patterned by surface-charge lithography.
PDMS is being used in the making of synthetic Gecko adhesion dry adhesive materials, to date only in laboratory test quantities.
Some flexible electronics researchers use PDMS because of its low cost, easy fabrication, flexibility, and optical transparency.
Medicine and cosmetics
Activated dimethicone, a mixture of polydimethylsiloxanes and silicon dioxide (sometimes called simethicone), is often used in over-the-counter drugs as an antifoaming agent and carminative.
Skin
PDMS is used variously in the cosmetic and consumer product industry as well. For example, PDMS can be used in the treatment of head lice on the scalp and dimethicone is used widely in skin-moisturizing lotions where it is listed as an active ingredient whose purpose is "skin protection." Some cosmetic formulations use dimethicone and related siloxane polymers in concentrations of use up to 15%. The Cosmetic Ingredient Review's (CIR) Expert Panel, has concluded that dimethicone and related polymers are "safe as used in cosmetic formulations."
Hair
PDMS compounds such as amodimethicone, are effective conditioners when formulated to consist of small particles and be soluble in water or alcohol/act as surfactants. (especially for damaged hair.) and are even more conditioning to the hair than common Dimethicone and/or Dimethicone copolyols.
Foods
PDMS can be found in many cooking oils and processed foods and fast food items such as McDonald's Chicken McNuggets and French fries, and Wendy's French fries.
Domestic and niche uses
Many people are indirectly familiar with PDMS because it is an important component in Silly Putty, to which PDMS imparts its characteristic viscoelastic properties. The rubbery, vinegary-smelling silicone caulks, adhesives, and aquarium sealants are also well-known. PDMS is also used as a component in silicone grease and other silicone based lubricants, as well as in defoaming agents, mold release agents, damping fluids, heat transfer fluids, polishes, cosmetics, hair conditioners and other applications. PDMS has also been used as a filler fluid in breast implants.
It can be used as a sorbent for the analysis of headspace (dissolved gas analysis) of food.
Safety and environmental considerations
According to Ullmann's Encyclopedia, no "marked harmful effects on organisms in the environment" have been noted for siloxanes. PDMS is nonbiodegradable, but is absorbed in waste water treatment facilities. Its degradation is catalyzed by various clays.
See also
- Silicone
- Cyclomethicone
- Siloxanes and other organosilicon compounds
- Polymethylhydrosiloxane (PMHS)
- Silicone rubber
References
- "Linear Polydimethylsiloxanes" Joint Assessment of Commodity Chemicals, September 1994 (Report No. 26) ISSN 0773-6339-26.
- ^ Mark, J. E.; Allcock, H. R.; West, R. “Inorganic Polymers” Prentice Hall, Englewood, NJ: 1992. ISBN 0-13-465881-7.
- Lotters, J. C.; Olthuis, W.; Veltink, P. H.; Bergveld, P. (1997). "The mechanical properties of the rubber elastic polymer polydimethylsiloxane for sensor applications". J Micromech Microeng. 7 (3): 145–147. doi:10.1088/0960-1317/7/3/017.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ McDonald, J. C.; et al. (2000). "Fabrication of microfluidic systems in poly(dimethylsiloxane)". Electrophoresis. 21 (1): 27–40. doi:10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. PMID 10634468.
{{cite journal}}: Explicit use of et al. in:|author=(help) - H. Hillborg, J.F. Ankner, U.W. Gedde, G.D. Smith, H.K. Yasuda and K. Wikstrom (2000). "Crosslinked polydimethylsiloxane exposed to oxygen plasma studied by neutron reflectometry and other surface specific techniques". Polymer. 41 (18): 6851–6863. doi:10.1016/S0032-3861(00)00039-2.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Lee, J. N.; Park, C.; Whitesides, G. M. (2003). "Solvent Compatibility of Poly(dimethylsiloxane)-Based Microfluidic Devices". Anal. Chem. 75 (23): 6544–6554. doi:10.1021/ac0346712. PMID 14640726.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Rainer Höfer, Franz Jost, Milan J. Schwuger, Rolf Scharf, Jürgen Geke, Josef Kresse, Herbert Lingmann, Rudolf Veitenhansl and Werner Erwied "Foams and Foam Control" Ullmann's Encyclopedia of Industrial Chemistry, 2000, Wiley-VCH, Weinheim. doi:10.1002/14356007.a11_465
- "Pulse Penetrant". Retrieved 3 March 2009.
- http://householdproducts.nlm.nih.gov/cgi-bin/household/brands?tbl=brands&id=21003001
- PDMS in microfluidics : a review and tutorial
- Waldner, Jean-Baptiste (2008). Nanocomputers and Swarm Intelligence. London: John Wiley & Sons. pp. 92–93. ISBN 1-84704-002-0.
- Rogers, J. A.; Nuzzo, R. G. (2005). "Recent progress in Soft Lithography. In". Materials Today. 8 (2): 50–56. doi:10.1016/S1369-7021(05)00702-9.
- S. Grilli, V. Vespini, P. Ferraro (2008). "Surface-charge lithography for direct pdms micro-patterning". Langmuir. 24 (23): 13262–13265. doi:10.1021/la803046j. PMID 18986187.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Inspired by Gecko Feet, UMass Amherst Scientists Invent Super-Adhesive Material. 16 Feb 2012, UMass Press Release
- Bowei Zhang, Quan Dong, Can E. Korman, Zhenyu Li, and Mona E. Zaghloul. "Flexible packaging of solid-state integrated circuit chips with elastomeric microfluidics".
- William E. Prentice, Michael L. Voight (2001). Techniques in musculoskeletal rehabilitation. McGraw-Hill Professional. p. 369. ISBN 0-07-135498-0.
- Richard H. Hunt, G. N. J. Tytgat, Axcan Pharma (1998). Helicobacter Pylori: Basic Mechanisms to Clinical Cure 1998. Springer. p. 447. ISBN 0-7923-8739-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Burgess, Ian F. (2009). "The mode of action of dimeticone 4% lotion against head lice, Pediculus capitis". BMC Pharmacology. 9: 3. doi:10.1186/1471-2210-9-3. PMC 2652450. PMID 19232080.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Nair, B; Cosmetic Ingredients Review Expert Panel (2003). "Final Report on the Safety Assessment of Stearoxy Dimethicone, Dimethicone, Methicone, Amino Bispropyl Dimethicone, Aminopropyl Dimethicone, Amodimethicone, Amodimethicone Hydroxystearate, Behenoxy Dimethicone, C24-28 Alkyl Methicone, C30-45 Alkyl Methicone, C30-45 Alkyl Dimethicone, Cetearyl Methicone, Cetyl Dimethicone, Dimethoxysilyl Ethylenediaminopropyl Dimethicone, Hexyl Methicone, Hydroxypropyldimethicone, Stearamidopropyl Dimethicone, Stearyl Dimethicone, Stearyl Methicone, and Vinyldimethicone". International Journal of Toxicology. 22 (2 Suppl): 11–35. doi:10.1177/1091581803022S204. PMID 14555417.
- Conditioning Agents for Hair and Skin, by Randy Schueller and Perry Romanowski, page 273, retrieved on 11 Nov 2014. Amodimethicone is recognized for its extremely robust conditioning and for its ability to form clear products when used in high-surfactant shampoos. Amodimethicone is a useful ingredient in conditioners, gels, mousses, and permanents, but its use in shampoos has proved troublesome due to interactions between the cationic and the anionic surfactants, which can result in compatibility problems. However, the amodimethicone emulsion can be made compatible in high-surfactant-level shampoos
- Principles of Polymer Science and Technology in Cosmetics and Personal Care, by E. Desmond Goddard & James V. Gruber, page 299, retrieved on 11 Nov 2014. Amodimethicone is typically an emulsion-polymerized polymer; however, utilizing linear processing technology amodimethicone fluids may be prepared as neat fluids, and then emulsified by a mechanical process as desired. The most widely utilized amodimethicone emulsions contain as the surfactant pair either (1) tallowtrimonium chloride (and) nonoxy- nol-10, or (2) cetrimonium chloride (and) trideceth-10 or -12. These "uncapped" amino- functional silicone compounds may be characterized by a linear or branched structure. In either case, amodimethicone polymers will undergo a condensation cure reaction during drying to form a somewhat durable elastomeric film on the hair, providing wet- and dry- combing benefits, lowering triboelectric charging effects, and increasing softness of the dry hair. They are excellent conditioning agents, often found in conditioners, mousses, setting lotions, and less frequently in 2-in-l shampoos
- Ingredients and Production of Cosmetics: Technology of Skin- and Hair-Care Products in Japan, Hiroshi Iwata and Kunio Shimada, Springer Science & Business Media, page 144, retrieved on 11 Nov 2014. Amodimethicone is the most widely used amino-modified silicone. It has an aminopropyl group attached to the methyl group of Dimethicone. Amodimethicone of various degrees of amino modification are available as well as those that have POP, POE, or an alkyl group attached. Amino-modified silicones are cationic and affinitive to hair keratin. They are particularly highly affinitive to damaged hair, which is anionic due to the presence of cysteic acid
- Handbook of Cosmetic Science and Technology, Fourth Edition, André O. Barel, Marc Paye, Howard I. Maibach, page 567, retrieved on 11 Nov 2014. ...and amodimethicone, which is an amino-substituted silicone and silicone quats, which contain permanently quaternized ammonium groups. In general, amodimethicones and silicone quats condition better than dimethicones, which condition better than dimethicone copolyols
- "McDonald's Food Facts: Ingredients" (PDF). McDonald's Restaurants of Canada Limited. 2013-09-08. p. 13.
- "Wendy's: Nutrition and ingredient information" (PDF). Wendy's International, Inc. September 2013. pp. 8–9.
- Micro Total Analysis Systems, Silly Putty, and Fluorous Peptides
- Headspace sorptive extraction (HSSE), stir bar sorptive extraction (SBSE), and solid phase microextraction (SPME) applied to the analysis of roasted Arabica coffee and coffee brew. Bicchi C1, Iori C, Rubiolo P and Sandra P, J Agric Food Chem. 30 January 2002, volume 50, issue 3, pages 449-459, PMID 11804511
- Hans-Heinrich Moretto, Manfred Schulze, Gebhard Wagner, "Silicones" Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a24_057
External links
- Amodimethicone Amodimethicone structure and properties
| E numbers | |
|---|---|
|
| Ectoparasiticides / arthropod (P03A) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insecticide/pediculicide |
| ||||||||||||||
| Acaricide/miticide/scabicide |
| ||||||||||||||
| |||||||||||||||