| Revision as of 23:26, 29 November 2006 editSrleffler (talk | contribs)Extended confirmed users, Pending changes reviewers, Rollbackers44,687 edits →Isotopes: spell out abbreviations.← Previous edit | Revision as of 23:28, 29 November 2006 edit undoSrleffler (talk | contribs)Extended confirmed users, Pending changes reviewers, Rollbackers44,687 edits →<sup>210</sup>Po: Pronouns in text should not refer to the section heading.Next edit → | ||
| Line 100: | Line 100: | ||
| === <sup>210</sup>Po === | === <sup>210</sup>Po === | ||
| Polonium-210 is an ] that has a half-life of 138.376 days. A milligram of <sup>210</sup>Po emits as many alpha particles as 5 grams of ]. A great deal of energy is released by its decay with half a gram quickly reaching a temperature above 750 K. A few ]s (1 curie equals 37 ]) of <sup>210</sup>Po emit a blue glow which is caused by ] of surrounding air. A single gram of <sup>210</sup>Po generates 140 watts of power.<ref>, Argonne National Laboratory</ref> Because it emits many ], which are stopped within a very short distance in dense media and release their energy, <sup>210</sup>Po has been used as a lightweight heat source to power ] in ]s. A <sup>210</sup>Po heat source was also used in each of the ] rovers deployed on the surface of the ], to keep their internal components warm during the lunar nights. Some anti-static brushes contain up to 500 microcuries of <sup>210</sup>Po as a source of charged particles for neutralizing static electricity in materials like photographic film.<ref>http://www.amstat.com/solutions/staticmaster.html</ref> The majority of the time <sup>210</sup>Po decays only by emission of an ], not by emission of an alpha particle and a ]. About one in a 100000 decays results in the emission of a gamma ray, this low gamma ray production rate makes it more difficult to find and identify this isotope. Rather than gamma ray spectroscopy, alpha spectroscopy will be the best method of measuring this isotope. | |||
| == Chemical characteristics == | == Chemical characteristics == | ||
Revision as of 23:28, 29 November 2006
Template:Elementbox header Template:Elementbox series Template:Elementbox groupperiodblock Template:Elementbox appearance Template:Elementbox atomicmass gpm Template:Elementbox econfig Template:Elementbox epershell Template:Elementbox section physicalprop Template:Elementbox phase Template:Elementbox density gpcm3nrt Template:Elementbox density gpcm3nrt Template:Elementbox meltingpoint Template:Elementbox boilingpoint Template:Elementbox heatfusion kjpmol Template:Elementbox heatvaporiz kjpmol Template:Elementbox heatcapacity jpmolkat25 Template:Elementbox vaporpressure katpa Template:Elementbox section atomicprop Template:Elementbox crystalstruct Template:Elementbox oxistates Template:Elementbox electroneg pauling Template:Elementbox ionizationenergies1 Template:Elementbox atomicradius pm Template:Elementbox atomicradiuscalc pm Template:Elementbox section miscellaneous Template:Elementbox magnetic Template:Elementbox eresist ohmmat0 Template:Elementbox thermalcond wpmkat300k Template:Elementbox thermalexpansion umpmkat25 Template:Elementbox cas number Template:Elementbox isotopes begin |- ! rowspan="2" style="text-align:right; vertical-align:middle;" | Po | rowspan="2" style="text-align:center; vertical-align:middle;" | syn | rowspan="2" style="text-align:right; vertical-align:middle;" | 2.898 y | α | style="text-align:right;" | Pb |- | ε, β | style="text-align:right;" | Bi |- ! rowspan="2" style="text-align:right; vertical-align:middle;" | Po | rowspan="2" style="text-align:center; vertical-align:middle;" | syn | rowspan="2" style="text-align:right; vertical-align:middle;" | 103 y | α | style="text-align:right;" | Pb |- | ε, β | style="text-align:right;" | Bi |- ! style="text-align:right;" | Po | style="text-align:center;" | syn | style="text-align:right;" | 138.376 d | α | style="text-align:right;" | Pb Template:Elementbox isotopes end Template:Elementbox footer
Polonium (IPA: /pə(ʊ)ˈləʊniəm/) is a chemical element in the periodic table that has the symbol Po and atomic number 84. A rare radioactive metalloid, polonium is chemically similar to tellurium and bismuth and occurs in uranium ores. Polonium has been studied for possible use in heating spacecraft. It exists as a number of isotopes.
Applications
When it is mixed or alloyed with beryllium, polonium can be a neutron source: beryllium releases a neutron upon absorption of an alpha particle that is supplied by Po. It has been used in this capacity as a neutron trigger for nuclear weapons. Other uses include:
- Devices that eliminate static charges in textile mills and other places. However, beta sources are more commonly used and are less dangerous. Another alternative is to use a high voltage direct current power supply to ionize air positively or negatively.
- Brushes that remove accumulated dust from photographic films. The polonium used in these brushes is sealed and controlled thus minimizing radiation hazards.
- As Po, a lightweight heat source to power thermoelectric cells.
History
Also called "Radium F", polonium was discovered by Marie Curie and her husband Pierre Curie in 1898 and was later named after Marie's homeland of Poland (Latin: Polonia). Poland at the time was under Russian, Prussian and Austrian domination, and not recognized as an independent country. It was Marie's hope that naming the element after her home land would add notoriety to its plight. Polonium may be the first element named to highlight a political controversy.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity. The pitchblende, after removal of uranium and radium, was more radioactive than both radium and uranium put together. This spurred them on to find the element. The electroscope showed it separating with bismuth.
Occurrence
A very rare element in nature, polonium is found in uranium ores at about 100 micrograms per metric ton (1:10). Its natural abundance is approximately 0.2% of the abundance of radium. Polonium has been found in tobacco smoke from tobacco leaves grown with phosphate fertilizers.
Synthesis by (n,g) reaction
In 1934 an experiment showed that when natural Bi is bombarded with neutrons, Bi, which is the parent of polonium, was created. Polonium may now be made in milligram amounts in this procedure which uses high neutron fluxes found in nuclear reactors. Only about 100 grams is produced each year, making polonium exceedingly rare.
Synthesis by (p,n) and (p,2n) reactions
It has been found that by proton bombardment of bismuth using a cyclotron that the longer lived isotopes of polonium can be formed. Other more proton rich isotopes can be formed by the irradation of platinium with carbon nuclei.
Isotopes
Polonium has 25 known isotopes all of which are radioactive. They have atomic masses that range from 194 u to 218 u. Po is the most widely available. Po (half-life 103 years) and Po (half-life 2.9 years) can be made through the alpha, proton, or deuteron bombardment of lead or bismuth in a cyclotron. However these isotopes are expensive to produce.
All elements containing 84 or more protons are radioactive. Alpha decay is a common form of decay for these nuclei. The most stable isotopes with more than 84 protons are thorium-232 and uranium-238; which form an "island of stability" which renders them stable enough to be found in large quantities in nature, but heavier nuclei are more and more affected by spontaneous fission.
Po
Polonium-210 is an alpha emitter that has a half-life of 138.376 days. A milligram of Po emits as many alpha particles as 5 grams of radium. A great deal of energy is released by its decay with half a gram quickly reaching a temperature above 750 K. A few curies (1 curie equals 37 gigabecquerels) of Po emit a blue glow which is caused by excitation of surrounding air. A single gram of Po generates 140 watts of power. Because it emits many alpha particles, which are stopped within a very short distance in dense media and release their energy, Po has been used as a lightweight heat source to power thermoelectric cells in artificial satellites. A Po heat source was also used in each of the Lunokhod rovers deployed on the surface of the Moon, to keep their internal components warm during the lunar nights. Some anti-static brushes contain up to 500 microcuries of Po as a source of charged particles for neutralizing static electricity in materials like photographic film. The majority of the time Po decays only by emission of an alpha particle, not by emission of an alpha particle and a gamma ray. About one in a 100000 decays results in the emission of a gamma ray, this low gamma ray production rate makes it more difficult to find and identify this isotope. Rather than gamma ray spectroscopy, alpha spectroscopy will be the best method of measuring this isotope.
Chemical characteristics
Polonium dissolves readily in dilute acids, but is only slightly soluble in alkalis. It is closely related chemically to bismuth and tellurium. Po (in common with ) has the ability to become airborne with ease: a 50% of a sample is vaporized in air in 45 hours at 328K (55°C, 131°F) even though its melting point is 527K (254°C, 489°F) and its boiling point is 1235K (962°C, 1763°F). More than one hypothesis exists for how polonium does this; one suggestion is that small clusters of polonium atoms are spalled off by the alpha decay.
It has been reported that microbes can methylate polonium by the action of methylcobalamin.
Solid state form
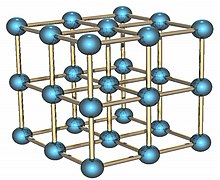
The alpha form of solid polonium is cubic with a distance of 3.352 Å between atoms. It is a simple cubic solid which is not interpenetrated.
The beta form of polonium is hexagonal; it has been reported in the chemical literature, along with the alpha form, several times.
Two papers report X-ray diffraction experiments on polonium metal. The first report of the crystal structure of polonium was done using electron diffraction.
Tests
Gamma counting

By means of radiometric methods such as gamma spectroscopy (or a method using a chemical separation followed by an activity measurement with a non-energy-dispersive counter), it is possible to measure the concentrations of radioisotopes and to distinguish one from another. In practice, background noise would be present and depending on the detector, the line width would be larger which would make it harder to identify and measure the isotope. In biological/medical work it is common to use the natural
Alpha counting
The best way to test for (and measure) many alpha emitters is to use alpha spectroscopy is it is common to place a drop of the test solution on a metal disk which is then dried out to give a uniform coating on the disk. This is then used as the test sample. If the thickness of the layer formed on the disk is too thick then the lines of the spectrum are broadened, this is becuase some of the energy of the alpha particles is lost during their movement through the layer of active material. An alternative method is to use internal liquid sintillation where the sample is mixed with a sintillation cocktail. Then the light emitted is then counted, some machines will record the amount of light energy per radioactive decay event. To show the effect of broadening on alpha spectra a series of graphs of simulated alpha spectra are shown below. From left to right the peaks are due to Po-209, Po-210, Pu-239 and Am-241. The fact that isotopes such as plutonium-239 and americium-241 have more than one alpha line indicates that the nucleous has the ability to be in different discrete energy levels (like a molecule can).



Toxicity
Polonium is a highly radioactive and toxic element and is very difficult to handle. Even in milligram or microgram amounts, handling Po is extremely dangerous, requiring specialized equipment and strict handling procedures. Alpha particles emitted by polonium will damage organic tissue easily if polonium is ingested, inhaled, or absorbed (though they do not penetrate the epidermis and hence are not hazardous if the polonium is outside the body).
The committed effective dose equivalent (CEDE) of 5.14×10 sieverts per becquerel (1.9×10 mrem/microcurie) for ingested Po, this value is vital for working out the cancer risk associated with Po. For an assessment of acute effects (radiation sickness) the dose rate (Gy day
In rats a dose of 1.45 MBq/kg of Po tends to cause death in about 30 days.
The maximum allowable body burden for ingested polonium is only 1,100 becquerels (0.03 microcurie), which is equivalent to a particle weighing only 6.8 × 10 gram. Weight for weight, polonium is approximately 2.5 × 10 (250 billion) times as toxic as hydrogen cyanide. The maximum permissible concentration for airborne soluble polonium compounds is about 7,500 Bq/m (2 × 10 µCi/cm). The biological halflife of polonium in humans is 30 to 50 days.
See also
- Isotopes of polonium
- The entry for polonium at fictional applications of real materials
- Alexander Litvinenko poisoning
References
- Curie P., Curie M. (1898). Comptes Rendus. 126: 1101.
- Pfützner M. (1999). "Borders of the Nuclear World --- 100 Years After Discovery of Polonium". Acta Physica Polonica B. 30: 1197.
{{cite journal}}: Text "issue 5" ignored (help) - Adloff J. P. (681–688). "The centennial of the 1903 Nobel Prize for physics". Radichimica Acta. 91: 2003. doi:10.1524/ract.91.12.681.23428.
{{cite journal}}: Text "issue 12" ignored (help)CS1 maint: date format (link) - Kabzinska K. (1998). "Chemical and Polish aspects of polonium and radium discovery". Przemysl Chemiczy. 77: 104–107.
{{cite journal}}: Text "issue 3" ignored (help) - Kilthau, Gustave F. "Cancer risk in relation to radioactivity in tobacco". Radiologic Technology. 67: 217–222.
{{cite journal}}: Unknown parameter|pim=ignored (help) - Alpha Radioactivity (210 Polonium) and Tobacco Smoke
- http://www.rsc.org/chemistryworld/News/2006/November/27110601.asp RSC Chemistry World Q&A
- Atterling, H., Forsling, W. (1959). "Light Polonium Isotopes from Carbon Ion Bombardments of Platinum". Arkiv for Fysik. 15 (1): 81–88.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Polonium, Argonne National Laboratory
- http://www.amstat.com/solutions/staticmaster.html
- Bogdan Wąs, Ryszard Misiak, Mirosław Bartyzel, Barbara Petelenz (2006). "Thermochromatographic Separation of ,Po from a Bismuth Target Bombardet with Protons" (PDF). Nukleonica. 51 (Suppl. 2): s3–s5.
{{cite journal}}: CS1 maint: multiple names: authors list (link) -
Momoshima N., Song L.X., Osaki S.,Maeda Y., (2001). "Formation and emission of volatile polonium compound by microbial activity and polonium methylation with methylcobalamin". Environ Sci Technol. 35 (15): 2956–2960. doi:10.1021/es001730+ S0013-936X(00)01730-2.
{{cite journal}}: Check|doi=value (help); Cite has empty unknown parameter:|1=(help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) -
Momoshima N., Song L.X., Osaki S.,Maeda Y., (2002). "Biologically induced Po emission from fresh water". J Environ Radioact. 63 (2): 187–197. doi:10.1016/S0265-931X(02)00028-0.
{{cite journal}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - R.J. Desando and R.C Lange, Journal of Inorganic and Nuclear Chemistry, 1966, 28, 1837-1846.
- W.H Beamer and C.R. Maxwell, Journal of Chemical Physics, 1946, 14, 569-569.
- M.A. Rollier, S.B. Hendricks and L.R. Maxwell, Journal of Chemical Physics, 1936, 4, 648-652.
- Nuclide Safety Data Sheet: Polonium–210
- Rencováa J., Svoboda V., Holuša R., Volf V., Jones M. M., Singh P. K. (1997). "Reduction of subacute lethal radiotoxicity of polonium-210 in rats by chelating agents". International Journal of Radiation Biology. 72 (3): 247–249. doi:10.1080/095530097143338.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Effective half-life of polonium in the human
External links
References and External links verified 2006-11-25 unless noted.
- WebElements.com – Polonium
- History of Polonium
- Los Alamos National Laboratory – Polonium
- NLM Hazardous Substances Databank – Polonium, Radioactive