| Revision as of 17:39, 2 April 2021 editLegionMammal978 (talk | contribs)Extended confirmed users7,894 editsm capitalized name← Previous edit | Revision as of 11:39, 16 March 2023 edit undoCitation bot (talk | contribs)Bots5,393,099 edits Alter: pages. Add: authors 1-1. Removed parameters. Some additions/deletions were parameter name changes. | Use this bot. Report bugs. | Suggested by Abductive | Category:Restricted titles | #UCB_Category 38/828Next edit → | ||
| Line 51: | Line 51: | ||
| }} | }} | ||
| '''Benzophenanthrene''' is a ] with the ] C<sub>18</sub>H<sub>12</sub>. It is a white solid that is soluble in nonpolar organic solvents. It is a nonplanar molecule<ref>{{Cite journal| |
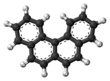
'''Benzophenanthrene''' is a ] with the ] C<sub>18</sub>H<sub>12</sub>. It is a white solid that is soluble in nonpolar organic solvents. It is a nonplanar molecule<ref>{{Cite journal|last1=Hirshfeld|first1=F. L.|last2=Sandler|first2=Selina|last3=Schmidt|first3=G. M. J.|date=1963-01-01|title=398. The structure of overcrowded aromatic compounds. Part VI. The crystal structure of benzophenanthrene and of 1,12-dimethylbenzophenanthrene|journal=Journal of the Chemical Society (Resumed)|language=en|pages=2108|doi=10.1039/jr9630002108|issn=0368-1769}}</ref><ref>{{cite journal |first1=M. |last1=Levy |first2= Melvin S. |last2= Newman |first3= M. |last3= Szwarc |title= Methyl Affinities of Non-planar Aromatic Hydrocarbons |journal= J. Am. Chem. Soc. |year= 1955 |volume= 77 |issue= 16 |pages= 4225 |doi= 10.1021/ja01621a015}}</ref> consisting of the fusion of four fused ] rings. The compound is of mainly theoretical interest but it is environmentally occurring<ref>{{Cite journal|last1=Tolosa|first1=Imma|last2=de Mora|first2=Stephen|last3=Sheikholeslami|first3=Mohammad Reza|last4=Villeneuve|first4=Jean-Pierre|last5=Bartocci|first5=Jean|last6=Cattini|first6=Chantal|date=2004-01-01|title=Aliphatic and aromatic hydrocarbons in coastal caspian Sea sediments|journal=Marine Pollution Bulletin|volume=48|issue=1–2|pages=44–60|doi=10.1016/S0025-326X(03)00255-8|pmid=14725875}}</ref> and weakly ]ic.<ref>{{Cite journal|last1=Hu|first1=X.|last2=Xia|first2=H.|last3=Srivastava|first3=S. K.|last4=Pal|first4=A.|last5=Awasthi|first5=Y. C.|last6=Zimniak|first6=P.|last7=Singh|first7=S. V.|date=1998-12-01|title=Catalytic efficiencies of allelic variants of human glutathione S-transferase P1-1 toward carcinogenic anti-diol epoxides of benzophenanthrene and benzochrysene|journal=Cancer Research|volume=58|issue=23|pages=5340–5343|issn=0008-5472|pmid=9850062}}</ref> | ||
| ==References== | ==References== | ||
Revision as of 11:39, 16 March 2023
Chemical compound The correct title of this article is Benzophenanthrene. The substitution of any brackets is due to technical restrictions.
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Benzophenanthrene | |||
| Other names 3,4-Benzophenanthrene; Benzophenanthrene; Tetrahelicene | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.362 | ||
| EC Number |
| ||
| KEGG | |||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C18H12 | ||
| Molar mass | 228.294 g·mol | ||
| Appearance | white solid | ||
| Density | 1.19 g/cm | ||
| Melting point | 68 °C (154 °F; 341 K) | ||
| Boiling point | 436.7 °C (818.1 °F; 709.8 K) @760mmHg | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |  
| ||
| Signal word | Warning | ||
| Hazard statements | H302, H312, H315, H319, H332, H335, H341, H351 | ||
| Precautionary statements | P201, P202, P261, P264, P270, P271, P280, P281, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P308+P313, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 | ||
| Flash point | 209.1 °C (408.4 °F; 482.2 K) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Benzophenanthrene is a polycyclic aromatic hydrocarbon with the chemical formula C18H12. It is a white solid that is soluble in nonpolar organic solvents. It is a nonplanar molecule consisting of the fusion of four fused benzene rings. The compound is of mainly theoretical interest but it is environmentally occurring and weakly carcinogenic.
References
- Hirshfeld, F. L.; Sandler, Selina; Schmidt, G. M. J. (1963-01-01). "398. The structure of overcrowded aromatic compounds. Part VI. The crystal structure of benzophenanthrene and of 1,12-dimethylbenzophenanthrene". Journal of the Chemical Society (Resumed): 2108. doi:10.1039/jr9630002108. ISSN 0368-1769.
- Levy, M.; Newman, Melvin S.; Szwarc, M. (1955). "Methyl Affinities of Non-planar Aromatic Hydrocarbons". J. Am. Chem. Soc. 77 (16): 4225. doi:10.1021/ja01621a015.
- Tolosa, Imma; de Mora, Stephen; Sheikholeslami, Mohammad Reza; Villeneuve, Jean-Pierre; Bartocci, Jean; Cattini, Chantal (2004-01-01). "Aliphatic and aromatic hydrocarbons in coastal caspian Sea sediments". Marine Pollution Bulletin. 48 (1–2): 44–60. doi:10.1016/S0025-326X(03)00255-8. PMID 14725875.
- Hu, X.; Xia, H.; Srivastava, S. K.; Pal, A.; Awasthi, Y. C.; Zimniak, P.; Singh, S. V. (1998-12-01). "Catalytic efficiencies of allelic variants of human glutathione S-transferase P1-1 toward carcinogenic anti-diol epoxides of benzophenanthrene and benzochrysene". Cancer Research. 58 (23): 5340–5343. ISSN 0008-5472. PMID 9850062.
| Polycyclic aromatic hydrocarbons | |
|---|---|
| 2 rings | |
| 3 rings | |
| 4 rings | |
| 5 rings | |
| 6 rings | |
| 7+ rings | |
| General classes | |
This article about a hydrocarbon is a stub. You can help Misplaced Pages by expanding it. |