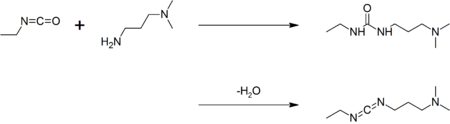| Revision as of 13:51, 7 July 2024 editIra Leviton (talk | contribs)Extended confirmed users331,449 editsm Fixed a PMC parameter in a citation. Please see Category:CS1 maint: PMC format.← Previous edit | Latest revision as of 14:12, 3 October 2024 edit undoAlchemization (talk | contribs)5 edits Added half sentence on how EDC is prepared with TsCl and TEA (with hyperlinks)Tag: Visual edit | ||
| Line 42: | Line 42: | ||
| ==Preparation== | ==Preparation== | ||
| EDC is commercially available. It may be prepared by coupling ethyl isocyanate to ''N'',''N''-dimethylpropane-1,3-diamine to give a ], followed by ]:<ref>{{cite journal | doi = 10.1021/jo01351a600 | year = 1961 |author1=Sheehan, John |author2=Cruickshank, Philip |author3=Boshart, Gregory | title =A Convenient Synthesis of Water-Soluble Carbodiimides | journal = ] | volume = 26 | pages = 2525 | issue = 7}}</ref> | EDC is commercially available. It may be prepared by coupling ethyl isocyanate to ''N'',''N''-dimethylpropane-1,3-diamine to give a ], followed by a ] mediated by ] and ]:<ref>{{cite journal | doi = 10.1021/jo01351a600 | year = 1961 |author1=Sheehan, John |author2=Cruickshank, Philip |author3=Boshart, Gregory | title =A Convenient Synthesis of Water-Soluble Carbodiimides | journal = ] | volume = 26 | pages = 2525 | issue = 7}}</ref> | ||
| :] | :] | ||
Latest revision as of 14:12, 3 October 2024

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 3-{amino}-N,N-dimethylpropan-1-amine | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.015.982 |
| PubChem CID | |
| UNII |
|
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H17N3 |
| Molar mass | 155.245 g·mol |
| Hazards | |
| Safety data sheet (SDS) | External MSDS (HCl Salt) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC, EDAC or EDCI) is a water-soluble carbodiimide usually handled as the hydrochloride.
It is typically employed in the 4.0-6.0 pH range. It is generally used as a carboxyl activating agent for the coupling of primary amines to yield amide bonds. While other carbodiimides like dicyclohexylcarbodiimide (DCC) or diisopropylcarbodiimide (DIC) are also employed for this purpose, EDC has the advantage that the urea byproduct formed (often challenging to remove in the case of DCC or DIC) can be washed away from the amide product using dilute acid. Additionally, EDC can also be used to activate phosphate groups in order to form phosphomonoesters and phosphodiesters. Common uses for this carbodiimide include peptide synthesis, protein crosslinking to nucleic acids, but also in the preparation of immunoconjugates. EDC is often used in combination with N-hydroxysuccinimide (NHS) for the immobilisation of large biomolecules. Recent work has also used EDC to assess the structure state of uracil nucleobases in RNA.
Preparation
EDC is commercially available. It may be prepared by coupling ethyl isocyanate to N,N-dimethylpropane-1,3-diamine to give a urea, followed by a dehydration reaction mediated by TsCl and TEA:
Mechanism
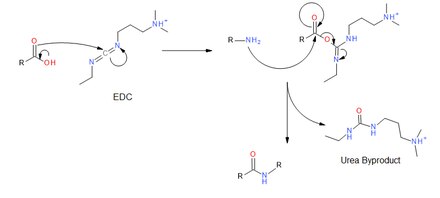
EDC couples primary amines, and other nucleophiles, to carboxylic acids by creating an activated ester leaving group. First, the carbonyl of the acid attacks the carbodiimide of EDC, and there is a subsequent proton transfer. The primary amine then attacks the carbonyl carbon of the acid which forms a tetrahedral intermediate before collapsing and discharging the urea byproduct. The desired amide is obtained.
Safety
In vivo dermal sensitization studies according to OECD 429 confirmed EDC is a strong skin sensitizer, showing a response at <0.01 wt% in the Local Lymph Node Assay (LLNA) placing it in Globally Harmonized System of Classification and Labelling of Chemicals (GHS) Dermal Sensitization Category 1A. Thermal hazard analysis by differential scanning calorimetry (DSC) shows EDC poses minimal explosion risks.
References
- Richard S. Pottorf, Peter Szeto (2001). "1-Ethyl-3-(3'-dimethylaminopropyl)carbodiimide Hydrochloride". E-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.re062.
- Mitchell, D; Renda, A; Douds, C; Babitzke, P; Assmann, S; Bevilacqua, P (2019). "In vivo RNA structural probing of uracil and guanine base-pairing by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC)". RNA. 25 (1): 147–157. doi:10.1261/rna.067868.118. PMC 6298566. PMID 30341176.
- Wang, PY; Sexton, AN; Culligan, WJ; Simon, MD (2019). "Carbodiimide reagents for the chemical probing of RNA structure in cells". RNA. 25 (1): 135–146. doi:10.1261/rna.067561.118. PMC 6298570. PMID 30389828.
- Sheehan, John; Cruickshank, Philip; Boshart, Gregory (1961). "A Convenient Synthesis of Water-Soluble Carbodiimides". J. Org. Chem. 26 (7): 2525. doi:10.1021/jo01351a600.
- Tsakos, Michail; Schaffert, Eva S.; Clement, Lise L.; Villadsen, Nikolaj L.; Poulsen, Thomas B. (2015). "Ester coupling reactions – an enduring challenge in the chemical synthesis of bioactive natural products". Natural Product Reports. 32 (4): 605–632. doi:10.1039/C4NP00106K. PMID 25572105.
- "Carbodiimide Crosslinker Chemistry - US". www.thermofisher.com. Retrieved 2019-05-10.
- OECD (2010). Test No. 429: Skin Sensitisation: Local Lymph Node Assay. Paris: Organisation for Economic Co-operation and Development.
- Graham, Jessica C.; Trejo-Martin, Alejandra; Chilton, Martyn L.; Kostal, Jakub; Bercu, Joel; Beutner, Gregory L.; Bruen, Uma S.; Dolan, David G.; Gomez, Stephen; Hillegass, Jedd; Nicolette, John; Schmitz, Matthew (2022-06-20). "An Evaluation of the Occupational Health Hazards of Peptide Couplers". Chemical Research in Toxicology. 35 (6): 1011–1022. doi:10.1021/acs.chemrestox.2c00031. ISSN 0893-228X. PMC 9214767. PMID 35532537.
- Sperry, Jeffrey B.; Minteer, Christopher J.; Tao, JingYa; Johnson, Rebecca; Duzguner, Remzi; Hawksworth, Michael; Oke, Samantha; Richardson, Paul F.; Barnhart, Richard; Bill, David R.; Giusto, Robert A.; Weaver, John D. (2018-09-21). "Thermal Stability Assessment of Peptide Coupling Reagents Commonly Used in Pharmaceutical Manufacturing". Organic Process Research & Development. 22 (9): 1262–1275. doi:10.1021/acs.oprd.8b00193. ISSN 1083-6160.
Further reading
- López-Alonso, JP; Diez-Garcia, F; Font, J; Ribó, M; Vilanova, M; Scholtz, JM; González, C; Vottariello, F; Gotte, G; Libonati, M; Laurents, DV (2009). "Carbodiimide EDC Induces Cross-Links That Stabilize RNase A C-dimer against Dissociation: EDC Adducts Can Affect Protein Net Charge, Conformation and Activity". Bioconjugate Chemistry. 20 (8): 1459–1473. doi:10.1021/bc9001486. PMID 19606852.
- Nakajima, N; Ikada, Y (1995). "Mechanism of Amide Formation by Carbodiimide for Bioconjugation in Aqueous Media". Bioconjugate Chemistry. 6 (1): 123–130. doi:10.1021/bc00031a015. PMID 7711098.