
In organic chemistry, a carbodiimide (systematic IUPAC name: methanediimine) is a functional group with the formula RN=C=NR. On Earth they are exclusively synthetic, but in interstellar space the parent compound HN=C=NH has been detected by its maser emissions.
A well known carbodiimide is dicyclohexylcarbodiimide, which is used in peptide synthesis. Dialkylcarbodiimides are stable. Some diaryl derivatives tend to convert to dimers and polymers upon standing at room temperature, though this mostly occurs with low melting point carbodiimides that are liquids at room temperature. Solid diaryl carbodiimides are more stable, but can slowly undergo hydrolysis in the presence of water over time.
Structure and bonding

From the perspective of bonding, carbodiimides are isoelectronic with carbon dioxide. Three principal resonance structures describe carbodiimides:
- RN=C=NR ↔ RN≡C-NR ↔ RN-C≡NR
The N=C=N core is relatively linear and the C-N=C angles approach 120°. In the case of C(NCHPh2)2, the central N=C=N angle is 170° and the C-N=C angles are within 1° of 126°. The C=N distances are short, nearly 120 pm, as is characteristic of double bonds. Carbodiimides are chiral, possessing C2-symmetry and therefore axial chirality. However, due to the low energy barrier to the molecule rotating and thereby converting quickly between its isomers, the actual isolation of one optical isomer of a carbodiimide is extremely difficult. It has been demonstrated at least once, in the case of conformationally restricted cyclic carbodiimides; though there are other reports of one-handed axially chiral carbodiimides, their validity has since been called into question on experimental and computational grounds.
The parent compound, methanediimine, (HN=C=NH), is a tautomer of cyanamide.
Synthesis
From thioureas and ureas
A classic route to carbodiimides involves dehydrosulfurization of thioureas. A typical reagent for this process is mercuric oxide:
- (R(H)N)2CS + HgO → (RN)2C + HgS + H2O
This reaction can often be conducted as stated, even though carbodiimides react with water. In some cases, a dehydrating agent is added to the reaction mixture.
The dehydration of N,N'-dialkylureas gives carbodiimides:
- (R(H)N)2CO → (RN)2C + H2O
Phosphorus pentoxide and p-Toluenesulfonyl chloride have been used as a dehydrating agents.
From isocyanates
Isocyanates can be converted to carbodiimides with loss of carbon dioxide:
- 2 RN=C=O → (RN)2C + CO2
The reaction is catalyzed by phosphine oxides. This reaction is reversible.
Reactions
Compared to other heteroallenes, carbodiimides are very weak electrophiles and only react with nucleophiles in the presence of catalysts, such as acids. In this way, guanidines can be prepared. As weak bases, carbodiimides bind to Lewis acids to give adducts.
Moffatt oxidation
Carbodiimides are reagents for the Moffatt oxidation, a protocol for conversion of an alcohol to a carbonyl (ketone or aldehyde) using dimethyl sulfoxide as the oxidizing agent:
- (CH3)2SO + (CyN)2C + R2CHOH → (CH3)2S + (CyNH)2CO + R2C=O
Typically the sulfoxide and diimide are used in excess. The reaction generates dimethyl sulfide and a urea as byproducts.
Coupling agents
In organic synthesis, compounds containing the carbodiimide functionality are used as dehydration agents. Specifically they are often used to convert carboxylic acids to amides or esters. Additives, such as N-hydroxybenzotriazole or N-hydroxysuccinimide, are often added to increase yields and decrease side reactions.
Polycarbodiimides can also be used as crosslinkers for aqueous resins, such as polyurethane dispersions or acrylic dispersion. Here the polycarbodiimide reacts with carboxylic acids, whose functional groups are often present in such aqueous resins, to form N-acyl urea. The result is the formation of covalent bonds between the polymer chains, making them crosslinked.
Amide formation pathway
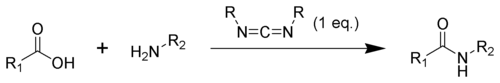
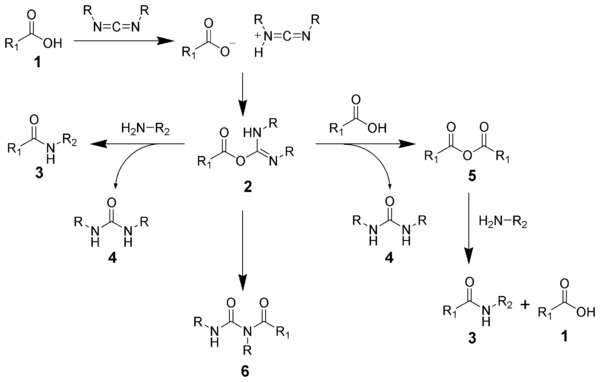
The formation of an amide using a carbodiimide is a common reaction, but carries the risk of several side reactions. The acid 1 will react with the carbodiimide to produce the key intermediate: the O-acylisourea 2, which can be viewed as a carboxylic ester with an activated leaving group. The O-acylisourea will react with amines to give the desired amide 3 and urea 4.
The possible reactions of the O-acylisourea 2 produce both desired and undesired products. The O-acylisourea 2 can react with an additional carboxylic acid 1 to give an acid anhydride 5, which can react further to give the amide 3. The main undesired reaction pathway involves the rearrangement of the O-acylisourea 2 to the stable N-acylurea 6. The use of solvents with low dielectric constants such as dichloromethane or chloroform can minimize this side reaction.
Examples
DCC

DCC (acronym for N,N'-dicyclohexylcarbodiimide) was one of the first carbodiimides developed as a reagent. It is widely used for amide and ester formation, especially for solid-phase synthesis of peptides. DCC has achieved popularity mainly because of its high-yielding amide coupling reactions and the fact that it is quite inexpensive.
However, DCC does have some serious drawbacks, and its use is often avoided for several reasons:
- The byproduct N,N'-dicyclohexylurea is mostly removed by filtration, but trace impurities can be difficult to remove. It is incompatible with traditional solid-phase peptide synthesis.
- DCC is a potent allergen, and repeated contact with skin increases the probability of sensitization to the compound. Clinical reports of individuals who cannot enter rooms where peptide coupling agents are used have been reported.
DIC

In contrast to DCC, DIC (N,N'-diisopropylcarbodiimide) is a liquid. Its hydrolysis product, N,N'-diisopropylurea, is soluble in organic solvents.
EDC
Main article: 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimideEDC is a water-soluble carbodiimide reagent used for a wide range of purposes. Apart from uses similar to those of DCC and DIC, it is also used for various biochemical purposes as a crosslinker or chemical probe.
CMCT or CMC
1-cyclohexyl-(2-morpholinoethyl)carbodiimide metho-p-toluene sulfonate is a carbodiimide developed for the chemical probing of RNA structure in biochemistry.
See also
- Sulfur diimide - the sulfur analogue
References
- Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 375. doi:10.1039/9781849733069-00372. ISBN 978-0-85404-182-4.
The name carbodiimide, for HN=C=NH, is retained but only for general nomenclature; no substitution of any kind is allowed. The systematic name, methanediimine, is the preferred IUPAC name.
- McGuire, Brett A.; Loomis, Ryan A.; Charness, Cameron M.; Corby, Joanna F.; Blake, Geoffrey A.; Hollis, Jan M.; Lovas, Frank J.; Jewell, Philip R.; Remijan, Anthony J. (2012-10-20). "Interstellar Carbodiimide (HNCNH): A New Astronomical Detection from the GBT Primos Survey Via Maser Emission Features". The Astrophysical Journal. 758 (2): L33. arXiv:1209.1590. Bibcode:2012ApJ...758L..33M. doi:10.1088/2041-8205/758/2/L33. ISSN 2041-8205. S2CID 26146516.
- ^ Andrew Williams; Ibrahim T. Ibrahim (1981). "Carbodiimide Chemistry: recent Advances". Chem. Rev. 81 (6): 589–636. doi:10.1021/cr00046a004.
- ^ T. W. Campbell; J. J. Monagle (1963). "Diphenylcarbodiimide". Org. Synth. 43: 31. doi:10.15227/orgsyn.043.0031.
- ^ Irngartinger, H.; Jäger, H.-U. (1978). "Kristall- und Molekularstrukturen von zwei Carbodiimiden: Bis(diphenylmethyl)carbodiimid und Bis(p-methoxyphenyl)-carbodiimid". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry. 34 (11): 3262–3265. doi:10.1107/S0567740878010626.
- Vincent, A. T.; Wheatley, P. J. (1972). "Crystal Structure of Bis-p-nitrophenylcarbodiimide, O2N·C6H4·N:C:N·C6H4·NO2". Journal of the Chemical Society, Perkin Transactions 2 (11): 1567–1571. doi:10.1039/P29720001567.
- Taniguchi, Tohru; Suzuki, Takahiro; Satoh, Haruka; Shichibu, Yukatsu; Konishi, Katsuaki; Monde, Kenji (2018). "Preparation of Carbodiimides with One-Handed Axial Chirality". Journal of the American Chemical Society. 140 (46): 15577−15581. doi:10.1021/jacs.8b08969. PMID 30398863. S2CID 53231838. Retrieved 18 August 2020.
- Damrauer, Robert; Lin, Hai; Damrauer, Niels H. (2014). "Computational Studies of Carbodiimide Rings". Journal of Organic Chemistry. 79 (9): 3781−3788. doi:10.1021/jo4026435. PMID 24716711. Retrieved 18 August 2020.
- ^ Frederick Kurzer; K. Douraghi-Zadeh (1967). "Advances in the Chemistry of Carbodiimides". Chem. Rev. 67 (2): ee107–152. doi:10.1021/cr60246a001. PMID 4859920.
- Henri Ulrich (2008). Chemistry and Technology of Carbodiimides. Wiley-VCH. ISBN 978-0-470-06510-5.
- John C. Sheehan; Philip A. Cruickshank (1968). "1-Ethyl-3-(3-Dimethylamino)propylcarbodiimide Hydrochloride and Methiodide". Org. Synth. 48: 83. doi:10.15227/orgsyn.048.0083.
- Arnab K. Maity; Skye Fortier; Leonel Griego; Alejandro J. Metta-Magaña (2014). "Synthesis of a "Super Bulky" Guanidinate Possessing an Expandable Coordination Pocket". Inorg. Chem. 53 (15): 8155–8164. doi:10.1021/ic501219q. PMID 25029088.
- Monagle, J. J. (1962). "Carbodiimides. III. Conversion of Isocyanates to Carbodiimides. Catalyst Studies". J. Org. Chem. 27 (11): 3851–3855. doi:10.1021/jo01058a022.
- Li, Zhen; Mayer, Robert J.; Ofial, Armin R.; Mayr, Herbert (2020-04-27). "From Carbodiimides to Carbon Dioxide: Quantification of the Electrophilic Reactivities of Heteroallenes". Journal of the American Chemical Society. 142 (18): 8383–8402. doi:10.1021/jacs.0c01960. PMID 32338511. S2CID 216557447.
- Tidwell, T. T. (1990). "Oxidation of Alcohols by Activated Dimethyl Sulfoxide and Related Reactions: An Update". Synthesis. 1990 (10): 857–870. doi:10.1055/s-1990-27036.
- John G. Moffatt (1967). "Cholane-24-al". Org. Synth. 47: 25. doi:10.15227/orgsyn.047.0025.
- Hesselmans, L. C. J.; Derksen, A. J.; van den Goorbergh, J. A. M. (2006). "Polycarbodiimide crosslinkers". Progress in Organic Coatings. 55 (2): 142–148. doi:10.1016/j.porgcoat.2005.08.011. ISSN 0300-9440.
- Posthumus, W.; Derksen, A. J.; van den Goorbergh, J. A. M.; Hesselmans, L. C. J. (2007). "Crosslinking by polycarbodiimides". Progress in Organic Coatings. 58 (2–3): 231–236. doi:10.1016/j.porgcoat.2006.09.031. ISSN 0300-9440.
- Hotan Mojarradi (2010). Coupling of substances containing a primary amine to hyaluronan via carbodiimide-mediated amidation (Thesis). Uppsala Universitet. ISSN 1650-8297.
 Media from Commons
Media from Commons Quotations from Wikiquote
Quotations from Wikiquote Textbooks from Wikibooks
Textbooks from Wikibooks Resources from Wikiversity
Resources from Wikiversity
