| Revision as of 18:09, 16 December 2024 editWallclockticking (talk | contribs)Extended confirmed users4,255 editsNo edit summaryTags: Mobile edit Mobile web edit Advanced mobile edit Newcomer task Newcomer task: copyedit← Previous edit | Revision as of 18:09, 16 December 2024 edit undoWallclockticking (talk | contribs)Extended confirmed users4,255 editsNo edit summaryTags: Mobile edit Mobile web edit Advanced mobile edit Newcomer task Newcomer task: copyeditNext edit → | ||
| Line 1: | Line 1: | ||
| {{Short description|Application of growing 3D tissue}} | {{Short description|Application of growing 3D tissue}} | ||
| {{Multiple issues| | |||
| {{peacock|date=February 2016}} | {{peacock|date=February 2016}} | ||
| }} | |||
| ] (HGBM) cells (indicated by the lower arrow) treated with magnetic nanoparticles were held at the air-medium interface by the magnetic field created by the magnet attached to the top of the first tissue culture plate. This image was taken after 48 hours of culturing.]] | ] (HGBM) cells (indicated by the lower arrow) treated with magnetic nanoparticles were held at the air-medium interface by the magnetic field created by the magnet attached to the top of the first tissue culture plate. This image was taken after 48 hours of culturing.]] | ||
| The '''Magnetic Levitation''' '''Method''' (MLM) is an emerging technique for ]. In this approach, cells are treated with magnetic ] and exposed to spatially varying ] generated by ] magnetic drivers. This process levitates the cells to the ] of a standard ]. The magnetic nanoparticle assemblies consist of magnetic ], ], and the ] ]. | The '''Magnetic Levitation''' '''Method''' (MLM) is an emerging technique for ]. In this approach, cells are treated with magnetic ] and exposed to spatially varying ] generated by ] magnetic drivers. This process levitates the cells to the ] of a standard ]. The magnetic nanoparticle assemblies consist of magnetic ], ], and the ] ]. | ||
Revision as of 18:09, 16 December 2024
Application of growing 3D tissue| This article contains wording that promotes the subject in a subjective manner without imparting real information. Please remove or replace such wording and instead of making proclamations about a subject's importance, use facts and attribution to demonstrate that importance. (February 2016) (Learn how and when to remove this message) |

The Magnetic Levitation Method (MLM) is an emerging technique for 3D cell culture. In this approach, cells are treated with magnetic nanoparticles and exposed to spatially varying magnetic fields generated by neodymium magnetic drivers. This process levitates the cells to the air/liquid interface of a standard petri dish. The magnetic nanoparticle assemblies consist of magnetic iron oxide nanoparticles, gold nanoparticles, and the polymer polylysine.
This method is scalable, accommodating cultures from as few as 500 cells to millions of cells, and is applicable in both single-dish systems and high-throughput low-volume systems. Magnetized culture cells can be used as building blocks (or "ink") for magnetic 3D bioprinting.
Overview
Hence, 3D cell culture methods have been developed to enable research into the behavior of cells in an environment that more accurately represents their interactions in vivo.
3D cell culturing by magnetic levitation uses biocompatible polymer-based reagents to deliver magnetic nanoparticles to individual cells so that an applied magnetic driver can levitate cells off the bottom of the cell culture dish and rapidly bring cells together near the air-liquid interface. This initiates cell-cell interactions in the absence of any artificial surface or matrix. Magnetic fields are designed to form 3D multicellular structures, including the expression of extracellular matrix proteins. The matrix, protein expression, and response to exogenous agents of resulting tissue show similarity to in-vivo results.
History
3D cell culturing by MLM was developed with collaboration between scientists at Rice University and University of Texas MD Anderson Cancer Center in 2008. 3D cell culturing technology was later licensed and commercialized by Nano3D Biosciences.
The magnetic levitation process

The figure on the right shows 3D cell culturing through magnetic levitation using one possible system. Letters on the figure refer to the following:
(A) A magnetic iron oxide nanoparticle assembly, known as the "nanoshuttle", is added and dispersed over cells, and the mixture is incubated.
(B) After incubation with the nanoshuttle, the cells are detached and transferred to a petri dish.
(C) A magnetic drive is then placed on top of a petri dish.
(D) The magnetic field causes cells to rise to the air–medium interface.
(E) Human umbilical vein endothelial cells (HUVEC) levitated for 60 minutes (left two images in E) and 4 hours (right two images in E) (scale bar: 50 μm).
The onset of cell-cell interaction takes place as soon as cells levitate, and 3D structures start to form. At 1 hour, the cells are still relatively dispersed, but they already show some signs of stretching. Formation of 3D structures is visible after 4 hours of levitation (arrows in E).
Protein expression
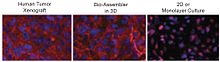
Patterns of protein expression in levitated cultures resemble the patterns observed in-vivo. For instance, as shown in the figure on the right, N-cadherin expression in levitated human glioblastoma (GBM) cells was similar to that seen in human tumor xenografts grown in immunodeficient mice (comparing the left and middle images), while standard 2D culture showed much weaker expression that did not match xenograft distribution (comparing the left and right images). The transmembrane protein N-cadherin is often used as an indicator of in vivo-like tissue assembly in 3D culturing.
Referring to the figure, in the mouse and levitated culture (left and middle image), N-cadherin is clearly concentrated in the membrane, and also present in cytoplasm and cell junctions, whereas the 2D system (right image) shows N-cadherin in the cytoplasm and nucleus, but notably absent from the membrane.
Applications

Co-culturing, magnetic manipulation, and invasion assays
One of the challenges of in vitro modelling of complex tissues is the difficulty of co-culturing different cell types. Co-culturing of different cell types can be achieved at the onset of levitation, either by mixing different cell types before levitation, or by magnetically guiding 3D cultures in an invasion assay format.
Co-culturing in a realistic tissue architecture is important for accurately modeling in vivo conditions, such as for increasing the accuracy of cellular assays, as shown in the figure on the right. In the figure, the human GBM cells and normal human astrocytes (NHA) are cultured separately and then magnetically guided together (left, time 0). Invasion of GBM into NHA in 3D culture provides an assay for basic cancer biology and drug screening (right, 12h to 252h).
Vascular simulation with stem cells
By facilitating the assembly of different populations of cells using the MLM, consistent generation of organoids termed adipospheres capable of simulating the complex intercellular interactions of endogenous white adipose tissue (WAT) can be achieved.
Co-culturing 3T3-L1 preadipocytes in 3D with murine endothelial bEND.3 cells create a vascular-like network assembly with concomitant lipogenesis in perivascular cells (refer to the attached figure).
In addition to cell lines, organogenesis of WAT (?) can be simulated from primary cells.
Adipocyte-depleted stromal vascular fraction (SVF) containing adipose stromal cells (ASC), endothelial cells, and infiltrating leukocytes derived from mouse WAT were cultured in 3D. This revealed organoids striking in hierarchical organization with distinct capsules and internal large vessel-like structures lined with endothelial cells, as well as perivascular localization of ASC.
Upon adipogenesis induction of either 3T3-L1 adipospheres or adipospheres derived from SVF, the cells efficiently formed large lipid droplets typical of white adipocytes in vivo, whereas only smaller lipid droplet formation is achievable in 2D. This indicates intercellular signaling that better recapitulates WAT organogenesis.
This MLM for 3D co-culturing creates a lipospheres appropriate for WAT modeling ex vivo and provides a new platform for functional screens to identify molecules bioactive toward individual adipose cell populations. It can also be adopted for WAT transplantation applications and aid other approaches to WAT-based cell therapy.
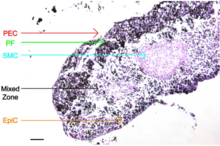
Organized co-culturing to create in vivo-like tissue
The use of additional manipulation tools may be needed to organize 3D co-cultures into a configuration similar enough to native tissue architecture.
Endothelial cells (PEC), smooth muscle cells (SMC), fibroblasts (PF), and epithelial cells (EpiC) cultured through magnetic levitation can be sequentially layered in a drag-and-drop manner to create bronchioles that maintain phenotype and induce extracellular matrix formation, as shown in the images on the right.
Cell types cultured
Listed below are the cell types (primary and cell lines) that have been successfully cultured by the magnetic levitation method.
| Cells | Cell line | Organism | Organ tissue | Image |
|---|---|---|---|---|
| Murine endothelial | Cell line | Mouse | Vessel | |
| Murine adipocyte | Cell line | Mouse | Adipose |  |
| Rattus norvegicus hepatoma | Cell line | Rat | Liver |  |
| Human pulmonary fibroblasts (HPF) | Primary | Human | Lung |  |
| Pulmonary endothelial (HPMEC) | Primary | Human | Lung | |
| Small airway epithelial (HSAEpiC) | Primary | Human | Lung |  |
| Bronchial epithelial | Primary | Human | Lung | |
| Human alveolar adenocarcinoma | A549 | Human | Lung |   |
| Type II alveolar | Primary | Human | Lung | |
| Human tracheal smooth muscle cells (HTSMCs) | Primary | Human | Lung |  |
| Human mesenchymal stem cells (HMSCs) | Primary | Human | Bone marrow |  |
| Human bone marrow endothelial cells (HBMECs) | Primary | Human | Bone marrow | |
| Dental pulp stem cells (DPSCs) | Primary | Human |  | |
| Human umbilical vein endothelial cells (HUVECs) | Primary | Human |  | |
| Murine chondrocytes | Primary | Mouse | Bone |  |
| Murine adipose tissue | Primary | Mouse |  | |
| Heart valve endothelial | Primary | Porcine | ||
| Pre-adipocytes fibroblasts | 3T3 | Mouse | ||
| Neural stem cells | C17.2 | Mouse | Brain |  |
| Human embryonic kidney cells | HEK293 | Human | Kidney |  |
| ] | B16 | Mouse | Skin | |
| Astrocytes | NHA | Human | Brain |  |
| Glioblastomas | LN229 | Human | Brain |  |
| T-cells and antigen presenting cells | Human | |||
| Mammary epithelial | MCF10A | Human | Breast |  |
| Breast cancer | MDA231 | Human | Breast | |
| Osteosarcoma | MG63 | Human | Bone |
References
- ^ Souza, G. R. et al. Three-dimensional Tissue Culture Based on Magnetic Cell Levitation. Nature Nanotechnol.5, 291-296, doi:10.1038/nnano.2010.23 (2010).
- ^ Molina, J., Hayashi, Y., Stephens, C. & Georgescu, M.-M. Invasive glioblastoma cells acquire stemness and increased Akt activation. Neoplasia12, 453-463 (2010).
- "Bio-Assembling in 3-D with Magnetic Levitation - Technology Review." Technology Review. N.p., n.d. Web. 20 Aug. 2012. <http://www.technologyreview.com/view/426363/bio-assembling-in-3-d-with-magnetic-levitation/>.
- "N3D Biosciences, Inc. » ABOUT US." N3D Biosciences, Inc. » ABOUT US. N.p., n.d. Web. 20 Aug. 2012. <http://www.n3dbio.com/about/ Archived 2013-12-14 at the Wayback Machine>.
- ^ Daquinag, A. C., Souza, G. R., Kolonin, M. G. Adipose Tissue Engineering in Three-Dimensional Levitation Tissue Culture System Based on Magnetic Nanoparticles. Tissue Eng. Part C. -Not available-, ahead of print. doi:10.1089/ten.tec.2012.0198 (2012).
- ^ Tseng, H., Gage, J. A., Raphael, R., Moore, R. H., Killian, T. C., Grande-Allen, K. J., Souza, G. R. Assembly of a three-dimensional multitype bronchiole co-culture model using magnetic levitation. Tissue Eng. Part C. -Not available-, ahead of print. doi:10.1089/ten.TEC.2012.0157 (2013)