| Revision as of 15:42, 27 December 2024 edit5.178.188.143 (talk) I will keep updating the article, and I DO NOT recommend you attempt a fourth revertTags: Reverted Visual edit: Switched← Previous edit | Revision as of 15:45, 27 December 2024 edit undoUtherSRG (talk | contribs)Autopatrolled, Administrators177,565 editsm Reverted edit by 5.178.188.143 (talk) to last version by MrOllieTags: Rollback RevertedNext edit → | ||
| Line 64: | Line 64: | ||
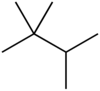
| '''Triptane''', or '''2,2,3-trimethylbutane''', is an ] ] with the molecular formula ]<sub>7</sub>]<sub>16</sub> or (H<sub>3</sub>C-)<sub>3</sub>C-C(-CH<sub>3</sub>)<sub>2</sub>H. It is therefore an ], specifically the most compact and heavily branched of the ] isomers, the only one with a ] (C<sub>4</sub>) backbone. | '''Triptane''', or '''2,2,3-trimethylbutane''', is an ] ] with the molecular formula ]<sub>7</sub>]<sub>16</sub> or (H<sub>3</sub>C-)<sub>3</sub>C-C(-CH<sub>3</sub>)<sub>2</sub>H. It is therefore an ], specifically the most compact and heavily branched of the ] isomers, the only one with a ] (C<sub>4</sub>) backbone. | ||
| Triptane is commonly used as an ] in ]s. | |||
| It was first synthesized in 1922 by Belgian chemists Georges Chavanne (1875-1941) and B. Lejeune, who called it '''trimethylisopropylmethane'''.<ref>{{Cite journal |last=Chavanne |first=G. |last2=Lejeune |first2=B. |date=March 1922 |title=Un nouvel heptane : le triméthylisopropylméthane |url=https://archive.org/details/v31_1922/v31_1922/page/n99/mode/1up |journal=Bulletin de la Société Chimique de Belgique |volume=31 |issue=3 |pages=99-102 |via=Internet Archive}}</ref><ref>https://webbook.nist.gov/cgi/cbook.cgi?Source=1922CHA%2FLEJ98</ref> | |||
| Due to its high ] (112-113 RON, 101 MON<ref>{{Cite journal |last=Nash |first=Connor P. |last2=Dupuis |first2=Daniel P. |last3=Kumar |first3=Anurag |last4=Farberow |first4=Carrie A. |last5=To |first5=Anh T. |last6=Yang |first6=Ce |last7=Wegener |first7=Evan C. |last8=Miller |first8=Jeffrey T. |last9=Unocic |first9=Kinga A. |last10=Christensen |first10=Earl |last11=Hensley |first11=Jesse E. |last12=Schaidle |first12=Joshua A. |last13=Habas |first13=Susan E. |last14=Ruddy |first14=Daniel A. |date=2022-02-01 |title=Catalyst design to direct high-octane gasoline fuel properties for improved engine efficiency |url=https://www.sciencedirect.com/science/article/abs/pii/S0926337321009267 |journal=Applied Catalysis B: Environmental |volume=301 |pages=120801 |doi=10.1016/j.apcatb.2021.120801 |issn=0926-3373}}</ref><ref>{{Cite journal |last=Perdih |first=A. |last2=Perdih |first2=F. |date=2006 |title=Chemical Interpretation of Octane Number |url=https://www.semanticscholar.org/paper/Chemical-Interpretation-of-Octane-Number-Perdih-Perdih/83a4bbf402f180a462c5c1e3feba600092fd6081 |journal=Acta Chimica Slovenica}}</ref>) triptane was produced on ] starting from 1943<ref>{{Cite web |last=stason.org |first=Stas Bekman: stas (at) |title=10.1 The myth of Triptane |url=https://stason.org/TULARC/vehicles/gasoline-faq/10-1-The-myth-of-Triptane.html |access-date=2024-11-16 |website=stason.org}}</ref> for use as an ] in ]. It was extensively researched for this role and received the modern name in the late 1930s in a joint laboratory of ], ], ] and the ].<ref>{{Cite book |last= |first= |url=https://books.google.com/books?id=9XwiAQAAMAAJ&pg=PA28 |title=Annual Report of the National Advisory Committee for Aeronautics |date=1938 |publisher=U.S. Government Printing Office |pages=28 |language=en}}</ref> | |||
| As of 2011, it wasn't a significant component of US automobile gasoline, present only in trace amounts (0.05-0.1%).<ref>{{cite web |date=2011 |title=Hydrocarbon Composition of Gasoline Vapor Emissions from Enclosed Fuel Tanks |url=https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P100GPED.TXT |website=nepis.epa.gov |publisher=United States Environmental Protection Agency}}</ref> | |||
| ==See also== | ==See also== | ||
Revision as of 15:45, 27 December 2024
Not to be confused with triptan, a type of anti-migraine drug, Tryptan, a trade name of tryptophan, or triplane.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 2,2,3-Trimethylbutane | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 1730756 | ||
| ChemSpider | |||
| ECHA InfoCard | 100.006.680 | ||
| EC Number |
| ||
| PubChem CID | |||
| UNII | |||
| UN number | 1206 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C7H16 | ||
| Molar mass | 100.205 g·mol | ||
| Appearance | Colorless liquid | ||
| Odor | Odorless | ||
| Density | 0.693 g mL | ||
| Melting point | −26 to −24 °C; −15 to −11 °F; 247 to 249 K | ||
| Boiling point | 80.8 to 81.2 °C; 177.3 to 178.1 °F; 353.9 to 354.3 K | ||
| Vapor pressure | 23.2286 kPa (at 37.7 °C) | ||
| Henry's law constant (kH) |
4.1 nmol Pa kg | ||
| Magnetic susceptibility (χ) | -88.36·10 cm/mol | ||
| Refractive index (nD) | 1.389 | ||
| Thermochemistry | |||
| Heat capacity (C) | 213.51 J K mol | ||
| Std molar entropy (S298) |
292.25 J K mol | ||
| Std enthalpy of formation (ΔfH298) |
−238.0 – −235.8 kJ mol | ||
| Std enthalpy of combustion (ΔcH298) |
−4.80449 – −4.80349 MJ mol | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |    
| ||
| Signal word | Danger | ||
| Hazard statements | H225, H302, H305, H315, H336, H400 | ||
| Precautionary statements | P210, P261, P273, P301+P310, P331 | ||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | −7 °C (19 °F; 266 K) | ||
| Autoignition temperature |
450 °C (842 °F; 723 K) | ||
| Explosive limits | 1–7% | ||
| Related compounds | |||
| Related alkanes | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |||
Triptane, or 2,2,3-trimethylbutane, is an organic chemical compound with the molecular formula C7H16 or (H3C-)3C-C(-CH3)2H. It is therefore an alkane, specifically the most compact and heavily branched of the heptane isomers, the only one with a butane (C4) backbone.
Triptane is commonly used as an anti-knock additive in aviation fuels.
See also
References
- "Triptan - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 11 March 2012.
This article about a hydrocarbon is a stub. You can help Misplaced Pages by expanding it. |