| Revision as of 22:08, 13 November 2007 edit216.138.75.50 (talk) misspelled 'including'← Previous edit | Revision as of 23:09, 13 November 2007 edit undoShrampes (talk | contribs)111 edits added some usage informationNext edit → | ||
| Line 7: | Line 7: | ||
| |} | |} | ||
| A '''polyurethane''', commonly abbreviated '''PU''', |
A '''polyurethane''', commonly abbreviated '''PU''', any ] consisting of a chain of ] units joined by ] links. PU offers the elasticity of rubber combined with the toughness and durability of metal. Because urethane is available in a very broad hardness range - eraser-soft to bowling-ball-hard - it combines the characteristics of rubber, plastic and metal. | ||
| Polyurethane polymers are formed by reacting a ] containing at least two ] ]s with another monomer containing at least two ] groups in the presence of a ]. Polyurethane formulations cover an extremely wide range of stiffness, hardness, and densities. These materials include low density flexible ] used in ] and bedding, low density rigid foam used for ], soft solid ] used for gel pads and print rollers, and hard solid plastics used as electronic instrument bezels and structural parts. Polyurethanes are widely used in high resiliency flexible foam seating, rigid foam insulation panels, microcellular foam ]s and ]s, durable elastomeric wheels and tires, electrical potting compounds, high performance ]s and sealants, ] ], seals, gaskets, ] underlay, and hard plastic parts. Polyurethane products are often called "urethanes". They should not be confused with the specific substance ], also known as ]. Polyurethanes are not produced from ethyl carbamate, nor do they contain it. | |||
| ==History== | ==History== | ||
Revision as of 23:09, 13 November 2007
| PU polymer formed by reacting a diisocyanate with a polyol |
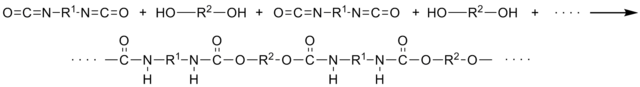
|
A polyurethane, commonly abbreviated PU, any polymer consisting of a chain of organic units joined by urethane links. PU offers the elasticity of rubber combined with the toughness and durability of metal. Because urethane is available in a very broad hardness range - eraser-soft to bowling-ball-hard - it combines the characteristics of rubber, plastic and metal.
Polyurethane polymers are formed by reacting a monomer containing at least two isocyanate functional groups with another monomer containing at least two alcohol groups in the presence of a catalyst. Polyurethane formulations cover an extremely wide range of stiffness, hardness, and densities. These materials include low density flexible foam used in upholstery and bedding, low density rigid foam used for thermal insulation, soft solid elastomers used for gel pads and print rollers, and hard solid plastics used as electronic instrument bezels and structural parts. Polyurethanes are widely used in high resiliency flexible foam seating, rigid foam insulation panels, microcellular foam seals and gaskets, durable elastomeric wheels and tires, electrical potting compounds, high performance adhesives and sealants, Spandex fibers, seals, gaskets, carpet underlay, and hard plastic parts. Polyurethane products are often called "urethanes". They should not be confused with the specific substance urethane, also known as ethyl carbamate. Polyurethanes are not produced from ethyl carbamate, nor do they contain it.
History
The pioneering work on polyurethane polymers was conducted by Otto Bayer and his coworkers in 1937 at the laboratories of I.G. Farben in Leverkusen, Germany. They recognized that using the polyaddition principle to produce polyurethanes from liquid diisocyanates and liquid polyether or polyester diols seemed to point to special opportunities, especially when compared to already existing plastics that were made by polymerizing olefins, or by polycondensation. The new monomer combination also circumvented existing patents obtained by Wallace Carothers on polyesters. Initially, work focused on the production of fibers and flexible foams. With development constrained by World War II (when PU's were applied on a limited scale as aircraft coating), it was not until 1952 that polyisocyanates became commercially available. Commercial production of flexible polyurethane foam began in 1954, based on toluene diisocyanate (TDI) and polyester polyols. The invention of these foams (initially called imitation swiss cheese by the inventors) was thanks to water accidentally introduced in the reaction mix. These materials were also used to produce rigid foams, gum rubber, and elastomers. Linear fibers were produced from hexamethylene diisocyanate (HDI) and 1,4-butanediol (BDO).
The first commercially available polyether polyol, poly(tetramethylene ether) glycol), was introduced by DuPont in 1956 by polymerizing tetrahydrofuran. Less expensive polyalkylene glycols were introduced by BASF and Dow Chemical the following year, 1957. These polyether polyols offered technical and commercial advantages such as low cost, ease of handling, and better hydrolytic stability; and quickly supplanted polyester polyols in the manufacture of polyurethane goods. Another early pioneer in PU's was the Mobay corporation. In 1960 more than 45,000 tons of flexible polyurethane foams were produced. As the decade progressed, the availability of chlorofluoroalkane blowing agents, inexpensive polyether polyols, and methylene diphenyl diisocyanate (MDI) heralded the development and use of polyurethane rigid foams as high performance insulation materials. Rigid foams based on polymeric MDI (PMDI) offered better thermal stability and combustion characteristics than those based on TDI. In 1967, urethane modified polyisocyanurate rigid foams were introduced, offering even better thermal stability and flammability resistance to low density insulation products. Also during the 1960s, automotive interior safety components such as instrument and door panels were produced by back-filling thermoplastic skins with semi-rigid foam.
In 1969, Bayer AG exhibited an all plastic car in Dusseldorf, Germany. Parts of this car were manufactured using a new process called RIM, Reaction Injection Molding. RIM technology uses high-pressure impingement of liquid components followed by the rapid flow of the reaction mixture into a mold cavity. Large parts, such as automotive fascia and body panels, can be molded in this manner. Polyurethane RIM evolved into a number of different products and processes. Using diamine chain extenders and trimerization technology gave poly(urethane urea), poly(urethane isocyanurate), and polyurea RIM. The addition of fillers, such as milled glass, mica, and processed mineral fibers gave arise to RRIM, reinforced RIM, which provided improvements in flexural modulus (stiffness) and thermal stability. This technology allowed production of the first plastic-body automobile in the United Sates, the Pontiac Fiero, in 1983. Further improvements in flexural modulus were obtained by incorporating preplaced glass mats into the RIM mold cavity, also known as SRIM, or structural RIM.
Starting in the early 1980s, water-blown microcellular flexible foam was used to mold gaskets for panel and radial seal air filters in the automotive industry. Since then, increasing energy prices and the desire to eliminate PVC plastisol from automotive applications have greatly increased market share. Costlier raw materials are offset by a significant decrease in part weight and in some cases, the elimination of metal end caps and filter housings. Highly filled polyurethane elastomers, and more recently unfilled polyurethane foams are now used in high-temperature oil filter applications.
Polyurethane foam (including foam rubber) is often made by adding small amounts of volatile materials, so-called blowing agents, to the reaction mixture. These simple volatile chemicals yield important performance characteristics, primarily thermal insulation. In the early 1990s, because of their impact on ozone depletion, the Montreal Protocol led to the greatly reduced use of many chlorine-containing blowing agents, such as trichlorofluoromethane (CFC-11). Other haloalkanes, such as the hydrochlorofluorocarbon 1,1-dichloro-1-fluoroethane (HCFC-141b), were used as interim replacements until their phase out under the IPPC directive on greenhouse gases in 1994 and by the Volatile Organic Compounds (VOC) directive of the EU in 1997 (See: Haloalkanes). By the late 1990s, the use of blowing agents such as carbon dioxide, pentane, 1,1,1,2-tetrafluoroethane (HFC-134a) and 1,1,1,3,3-pentafluoropropane (HFC-245fa) became more widespread in North America and the EU, although chlorinated blowing agents remained in use in many developing countries.
Building on existing polyurethane spray coating technology and polyetheramine chemistry, extensive development of two-component polyurea spray elastomers took place in the 1990s. Their fast reactivity and relative insensitivity to moisture make them useful coatings for large surface area projects, such as secondary containment, manhole and tunnel coatings, and tank liners. Excellent adhesion to concrete and steel is obtained with the proper primer and surface treatment. During the same period, new two-component polyurethane and hybrid polyurethane-polyurea elastomer technology was used to enter the marketplace of spray-in-place load bed liners. This technique for coating pickup truck beds and other cargo bays creates a durable, abrasion resistant composite with the metal substrate, and eliminates corrosion and brittleness associated with drop-in thermoplastic bed liners.
The use of polyols derived from vegetable oils to make polyurethane products began garnishing attention beginning around 2004, partly due to the rising costs of petrochemical feedstocks and partially due to an enhanced public desire for environmentally friendly green products. One of the most vocal supporters of these polyurethanes made using natural oil polyols is the Ford Motor Company.
Chemistry
| generalized polyurethane reaction |
Polyurethanes are in the class of compounds called reaction polymers, which include epoxies, unsaturated polyesters, and phenolics. A urethane linkage is produced by reacting an isocyanate group, -N=C=O with a hydroxyl (alcohol) group, -OH. Polyurethanes are produced by the polyaddition reaction of a polyisocyanate with a polyalcohol (polyol) in the presence of a catalyst and other additives. In this case, a polyisocyanate is a molecule with two or more isocyanate functional groups, R-(N=C=O)n ≥ 2 and a polyol is a molecule with two or more hydroxyl functional groups, R'-(OH)n ≥ 2. The reaction product is a polymer containing the urethane linkage, -RNHCOOR'-. Isocyanates will react with any molecule that contains an active hydrogen. Importantly, isocyanates react with water to form a urea linkage and carbon dioxide gas; they also react with polyetheramines to form polyureas. Commercially, polyurethanes are produced by reacting a liquid isocyanate with a liquid blend of polyols, catalyst, and other additives. These two components are referred to as a polyurethane system, or simply a system. The isocyanate is commonly referred to in North America as the 'A-side' or just the 'iso'. The blend of polyols and other additives is commonly referred to as the 'B-side' or as the 'poly'. This mixture might also be called a 'resin' or 'resin blend'. In Europe the meanings for 'A-side' and 'B-side' are reversed. Resin blend additives may include chain extenders, cross linkers, surfactants, flame retardants, blowing agents, pigments, and fillers.
The first essential component of a polyurethane polymer is the isocyanate. Molecules that contain two isocyanate groups are called diisocyanates. These molecules are also referred to as monomers or monomer units, since they themselves are used to produce polymeric isocyanates that contain three or more isocyanate functional groups. Isocyanates can be classed as aromatic, such as diphenylmethane diisocyanate (MDI) or toluene diisocyanate (TDI); or aliphatic, such as hexamethylene diisocyanate (HDI) or isophorone diisocyanate (IPDI). An example of a polymeric isocyanate is polymeric diphenylmethane diisocyanate, which is a blend of molecules with two-, three-, and four- or more isocyanate groups, with an average functionality of 2.7. Isocyanates can be further modified by partially reacting them with a polyol to form a prepolymer. A quasi-prepolymer is formed when the stoichiometric ratio of isocyanate to hydroxyl groups is greater than 2:1. A true prepolymer is formed when the stoichiometric ratio is equal to 2:1. Important characteristics of isocyanates are their molecular backbone, % NCO content, functionality, and viscosity.
The second essential component of a polyurethane polymer is the polyol. Molecules that contain two hydroxyl groups are called diols, those with three hydroxyl groups are called triols, et cetera. In practice, polyols are distinguished from short chain or low-molecular weight glycol chain extenders and cross linkers such as ethylene glycol (EG), 1,4-butanediol (BDO), diethylene glycol (DEG), glycerine, and trimethylol propane (TMP). Polyols are polymers in their own right. They are formed by free radical addition of propylene oxide (PO), ethylene oxide (EO) onto a hydroxyl or amine containing initiator, or by polyesterification of a di-acid, such as adipic acid, with glycols, such as ethylene glycol or dipropylene glycol (DPG). Polyols extended with PO or EO are polyether polyols. Polyols formed by polyesterification are polyester polyols. The choice of initiator, extender, and molecular weight of the polyol greatly affect its physical state, and the physical properties of the polyurethane polymer. Important characteristics of polyols are their molecular backbone, initiator, molecular weight, % primary hydroxyl groups, functionality, and viscosity.
| PU reaction mechanism catalyzed by a tertiary amine |

|
| carbon dioxide gas formed by reacting water and isocyanate |

|
The polymerization reaction is catalyzed by tertiary amines, such as dimethylcyclohexylamine, and organometallic compounds, such as dibutyltin dilaurate. Furthermore, catalysts can be chosen based on whether they favor the urethane (gel) reaction, such as 1,4-diazabicyclooctane (also called DABCO or TEDA), or the urea (blow) reaction, such as bis-(2-dimethylaminoethyl)ether, or specifically drive the isocyanate trimerization reaction, such as potassium octoate.
One of the most desirable attributes of polyurethanes is their ability to be turned into foam. Blowing agents such as water, certain halocarbons such as HFC-245fa (1,1,1,3,3-pentafluoropropane) and HFC-134a (1,1,1,2-tetrafluoroethane), and hydrocarbons such as n-pentane, can be incorporated into the poly side or added as an auxiliary stream. Water reacts with the isocyanate to create carbon dioxide gas, which fills and expands cells created during the mixing process. The reaction is a three step process. A water molecule reacts with an isocyanate group to form a carbamic acid. Carbamic acids are unstable, and decompose forming carbon dioxide and an amine. The amine reacts with more isocyanate to give a substituted urea. Water has a very low molecular weight, so even though the weight percent of water may be small, the molar proportion of water may be high and considerable amounts of urea produced. The urea is not very soluble in the reaction mixture and tends to form separate "hard segment" phases consisting mostly of polyurea. The concentration and organization of these polyurea phases can have a significant impact on the properties of the polyurethane foam. Halocarbons and hydrocarbons are chosen such that they have boiling points at or near room temperature. Since the polymerization reaction is exothermic, these blowing agents volatilize into a gas during the reaction process. They fill and expand the cellular polymer matrix, creating a foam. It is important to know that the blowing gas does not create the cells of a foam. Rather, foam cells are a result of blowing gas diffusing into bubbles that are nucleated or stirred into the system at the time of mixing. In fact, high density microcellular foams can be formed without the addition of blowing agents by mechanically frothing or nucleating the polyol component prior to use.
Surfactants are used to modify the characteristics of the polymer during the foaming process. They are used to emulsify the liquid components, regulate cell size, and stabilize the cell structure to prevent collapse and surface defects. Rigid foam surfactants are designed to produce very fine cells and a very high closed cell content. Flexible foam surfactants are designed to stabilize the reaction mass while at the same time maximizing open cell content to prevent the foam from shrinking. The need for surfactant can be affected by choice of isocyanate, polyol, component compatibility, system reactivity, process conditions and equipment, tooling, part shape, and shot weight.
Raw Materials
For the manufacture of polyurethane polymers, two groups of at least bifunctional substances are needed as reactants; compounds with isocyanate groups, and compounds with active hydrogen atoms. The physical and chemical character, structure, and molecular size of these compounds influence the polymerization reaction, as well as ease of processing and final physical properties of the finished polyurethane. In addition, additive such as catalysts, surfactants, blowing agents, cross linkers, flame retardants, light stabilizers, and fillers are used to control and modify the reaction process and performance characteristics of the polymer.
Isocyanates
Isocyanates with two or more functional groups are required for the formation of polyurethane polymers. Volume wise, aromatic isocyanates account for the vast majority of global diisocyanate production. Aliphatic and cycloaliphatic isocyanates are also important building blocks for polyurethane materials, but in much smaller volumes. There are a number of reasons for this. First, the aromatically linked isocyanate group is much more reactive than the aliphatic one. Second, aromatic isocyanates are more economical to use. Aliphatic isocyanates are used only if special properties are required for the final product. For example, light stable coatings and elastomers can only be obtained with aliphatic isocyanates. Even within the same class of isocyanates, there is a significant difference in reactivity of the functional groups based on steric hindrance. In the case of 2,4-toluene diisocyanate, the isocyanate group in the para position to the methyl group is much more reactive than the isocyanate group in the ortho position.
Phosgenation of corresponding amines is the main technical process for the manufacture of isocyanates. The amine raw materials are generally manufactured by the hydrogenation of corresponding nitro compounds. For example, toluenediamine (TDA) is manufactured from dinitrotoluene, which then converted to toluene diisocyanate (TDI). Diamino diphenylmethane or methylenedianiline (MDA) is manufactured from nitrobenzene via aniline, which is then converted to diphenylmethane diisocyanate (MDI).
The two most important aromatic isocyanates are toluene diisocyanate (TDI) and diphenylmethane diisocyanate (MDI). TDI consists of a mixture of the 2,4- and 2,6-diisocyantotoluene isomers. The most important product is TDI-80 (TD-80), consisting of 80% of the 2,4-isomer and 20% of the 2,6-isomer. This blend is used extensively in the manufacture of polyurethane flexible slabstock and molded foam. TDI, and especially crude TDI and TDI/MDI blends can be used in rigid foam applications, but have been supplanted by polymeric MDI. TDI-polyether and TDI-polyester prepolymers are used in high performance coating and elastomer applications. Prepolymers are available that have been vacuum stripped of TDI monomer, which greatly reduces their toxicity. Diphenylmethane diisocyanate (MDI) has three isomers, 4,4'-MDI, 2,4'-MDI, and 2,2'-MDI, and is also polymerized to provide oligomers of functionality three and higher.
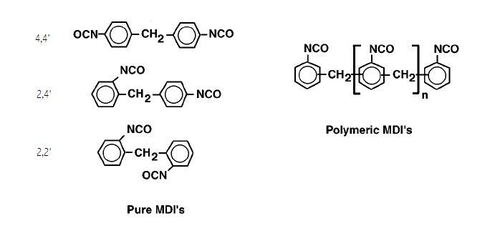
Only the 4,4'-MDI monomer is sold commercially as a single isomer. It is provided either as a frozen solid or flake, or in molten form, and is used to manufacture high performance prepolymers. Monomer blends, consisting of approximately 50% of the 4,4'-isomer and 50% of the 2,4'-isomer, are liquid at room temperature and are used to manufacture prepolymers for polyurea spray elastomer applications. 4,4'-MDI blends containing MDI uretonimine, carbodiimide, and allophonate moieties are also liquid at room temperature, and are used in the manufacture of integral skin and microcellular foams. 4,4'-MDI-glycol prepolymers offer increased mechanical properties in the same applications, but are prone to freezing at temperatures below 20°C. Polymeric MDI (PMDI) is used in rigid pour-in-place, spray foam, and molded foam applications. Polymeric MDI that contains a very high portion of high-functionality oligomers is used to manufacture polyurethane and polyisocyanurate rigid insulation boardstock. Modified PMDI, which contains high levels of MDI monomer, is used in the production of polyurethane flexible molded and microcellular foam. The relative percentage of the 4,4'- and 2,4'- isomers is adjusted to change the reactivity and storage stability of the isocyanate blend, as well as the firmness and other physical properties of the finished goods. Other aromatic isocyanate include p-phenylene diisocyante (PPDI), naphthalene diisocyanate (NDI), and o-tolidine diisocyanate (TODI).
The most important aliphatic and cycloaliphatic isocyanates are 1,6-hexamethylene diisocyanate (HDI), 1-isocyanato-3-isocyanatomethyl-3,5,5-trimethyl-cyclohexane (isophorone diisocyanate, IPDI), and 4,4'-diisocyanato dicyclohexylmethane (H12MDI). They are used to produce light stable, non-yellowing polyurethane coatings and elastomers. Because of their toxicity, aliphatic isocyanate monomers are converted into prepolymers, biurets, dimers, and trimers for commercial use. HDI adducts are used extensively for weather and abrasion resistant coatings and lacquers. IPDI is used in the manufacture of coatings, elastomeric adhesives and sealants. H12MDI prepolymers are used to produce high performance coatings and elastomers with optical clarity and hydrolysis resistance. Other aliphatic isocyanates include cyclohexane diisocyanate (CHDI), tetramethylxylene diisocyanate (TMXDI), and 1,3-bis(isocyanatomethyl)cyclohexane (H6XDI).
Polyols
Polyols are higher molecular weight materials manufactured from an initiator and monomeric building blocks. They are most easily classified as polyether polyols, which are made by the reaction of epoxides (oxiranes) with an active hydrogen containing starter compounds, or polyester polyols, which are made by the polycondensation of multifunctional carboxylic acids and hydroxyl compounds. They can be further classified according to their end use as flexible or rigid polyols, depending on the functionality of the initiator and their molecular weight. Taking into account functionality, flexible polyols have molecular weights from 2,000 to 10,000 (OH# from 18 to 56). Rigid polyols have molecular weights from 250 to 700 (OH# from 300 to 700). Polyols with molecular weights from 700 to 2,000 (OH# 60 to 280) are used to add stiffness or flexibility to base systems, as well as increase solubility of low molecular weight glycols in high molecular weight polyols.
Polyether polyols come in a wide variety of grades based on their end use, but are all constructed in a similar manner. Polyols for flexible applications use low functionality initiators such as dipropylene glycol (f=2) or glycerine (f=3). Polyols for rigid applications use high functionality initiators such sucrose (f=8), sorbitol (f=6), toluenediamine (f=4), and Mannich bases (f=4). Propylene oxide is then added to the initiators until the desired molecular weight is achieved. Polyols extended with propylene oxide are terminated with secondary hydroxyl groups. In order to change the compatibility, rheological properties, and reactivity of a polyol, ethylene oxide is used as a co-reactant to create random or mixed block heteropolymers. Polyols capped with ethylene oxide contain a high percentage of primary hydroxyl groups, which are more reactive than secondary hydroxyl groups. Because of their high viscosity (470 OH# sucrose polyol, 33,000 cps at 25°C), carbohydrate initiated polyols often use glycerine or diethylene glycol as a co-initiate in order to lower the viscosity to ease handling and processing (490 OH# sucrose-glycerine polyol, 5,500 cps at 25°C). Graft polyols (also called filled polyols or polymer polyols) contain finely dispersed styrene-acrylonitrile, acrylonitrile, or polyurea (PHD) polymer solids chemically grafted to a high molecular weight polyether backbone. They are used to increase the load bearing properties of low density high-resiliency (HR) foam, as well as add toughness to microcellular foams and cast elastomers. PHD polyols are also used to modify the combustion properties of HR flexible foam. Solids content ranges from 14% to 50%, with 22% and 43% being typical. Initiators such as ethylenediamine and triethanolamine are used to make low molecular weight rigid foam polyols that have built-in catalytic activity due to the presence of nitrogen atoms in the backbone. They are used to increase system reactivity and physical property build, and to reduce the friability of rigid foam molded parts. A special class of polyether polyols, poly(tetramethylene ether) glycols are made by polymerizing tetrahydrofuran. They are used in high performance coating and elastomer applications.
Polyester polyols fall into two distinct categories according to composition and application. Conventional polyester polyols are based on virgin raw materials and are manufactured by the direct polyesterification of high-purity diacids and glycols, such as adipic acid and 1,4-butanediol. They are distinguished by the choice of monomers, molecular weight, and degree of branching. While costly and difficult to handle because of their high viscosity, they offer physical properties not obtainable with polyether polyols, including superior solvent, abrasion, and cut resistance. Other polyester polyols are based on reclaimed raw materials. They are manufactured by transesterification (glycolysis) of recycled poly(ethyleneterephthalate) (PET) or dimethylterephthalate (DMT) distillation bottoms with glycols such as diethylene glycol. These low molecular weight, aromatic polyester polyols are used in the manufacture of rigid foam, and bring low cost and excellent flammability characteristics to polyisocyanurate (PIR) boardstock and polyurethane spray foam insulation.
Specialty polyols include polycarbonate polyols, polycaprolactone polyols, polybutadiene polyols, and polysulfide polyols. The materials are used in elastomer, sealant, and adhesive applications that require superior weatherability, and resistance to chemical and environmental attack. Natural oil polyols derived from castor oil and other vegetable oils are used to make elastomers, flexible bunstock, and flexible molded foam.
Chain extenders and cross linkers
Chain extenders (f=2) and cross linkers (f=3 or greater) are low molecular weight hydroxyl and amine terminated compounds that play an important role in the polymer morphology of polyurethane fibers, elastomers, adhesives, and certain integral skin and microcellular foams. The elastomeric properties of these materials are derived from the phase separation of the hard and soft copolymer segments of the polymer, such that the urethane hard segment domains serve as cross-links between the amorphous polyether (or polyester) soft segment domains. This phase separation occurs because the mainly non-polar, low melting soft segments are incompatible with the polar, high melting hard segments. The soft segments, which are formed from high molecular weight polyols, are mobile and are normally present in coiled formation, while the hard segments, which are formed from the isocyanate and chain extenders, are stiff and immobile. Because the hard segments are covalently coupled to the soft segments, they inhibit plastic flow of the polymer chains, thus creating elastomeric resiliency. Upon mechanical deformation, a portion of the soft segments are stressed by uncoiling, and the hard segments become aligned in the stress direction. This reorientation of the hard segments and consequent powerful hydrogen bonding contributes to high tensile strength, elongation, and tear resistance values. The choice of chain extender also determines flexural, heat, and chemical resistance properties. The most important chain extenders are ethylene glycol, 1,4-butanediol (1,4-BDO or BDO), 1,6-hexanediol, cyclohexane dimethanol and hydroquinone bis(2-hydroxyethyl) ether (HQEE). All of these glycols form polyurethanes that phase separate well and form well defined hard segment domains, and are melt processable. They are all suitable for thermoplastic polyurethanes with the exception of ethylene glycol, since the its derived bis-phenyl urethane undergoes unfavorable degradation at high hard segment levels. Diethanolamine and triethanolamine are used in flex molded foams to build firmness and add catalytic activity. Diethyltoluenediamine is used extensively in RIM, and in polyurethane and polyurea elastomer formulations.
| hydroxyl compounds – difunctional molecules | ||||
|---|---|---|---|---|
| MW | s.g. | f.p. °C | b.p. °C | |
| ethylene glycol | 62.1 | 1.110 | -13.4 | 197.4 |
| diethylene glycol | 106.1 | 1.111 | -8.7 | 245.5 |
| triethylene glycol | 150.2 | 1.120 | -7.2 | 287.8 |
| tetraethylene glycol | 194.2 | 1.123 | -9.4 | 325.6 |
| propylene glycol | 76.1 | 1.032 | supercools | 187.4 |
| dipropylene glycol | 134.2 | 1.022 | supercools | 232.2 |
| tripropylene glycol | 192.3 | 1.110 | supercools | 265.1 |
| 1,3-propanediol | 76.1 | 1.060 | -28 | 210 |
| 1,3-butanediol | 92.1 | 1.005 | - | 207.5 |
| 1,4-butanediol | 92.1 | 1.017 | 20.1 | 235 |
| neopentyl glycol | 104.2 | - | 130 | 206 |
| 1,6-hexanediol | 118.2 | 1.017 | 43 | 250 |
| 1,4-cyclohexanedimethanol | - | - | - | - |
| HQEE | - | - | - | - |
| ethanolamine | 61.1 | 1.018 | 10.3 | 170 |
| diethanolamine | 105.1 | 1.097 | 28 | 271 |
| methyldiethanolamine | 119.1 | 1.043 | -21 | 242 |
| phenyldiethanolamine | 181.2 | - | 58 | 228 |
| hydroxyl compounds – trifunctional molecules | ||||
| MW | s.g. | f.p. °C | b.p. °C | |
| glycerol | 92.1 | 1.261 | 18.0 | 290 |
| trimethylolpropane | - | - | - | - |
| 1,2,6-hexanetriol | - | - | - | - |
| triethanolamine | 149.2 | 1.124 | 21 | - |
| hydroxyl compounds – tetrafunctional molecules | ||||
| MW | s.g. | f.p. °C | b.p. °C | |
| pentaerythritol | 136.2 | - | 260.5 | - |
| N,N,N',N'-tetrakis (2-hydroxypropyl) ethylenediamine |
- | - | - | - |
| amine compounds – difunctional molecules | ||||
| MW | s.g. | f.p. °C | b.p. °C | |
| diethyltoluenediamine | 178.3 | 1.022 | - | 308 |
| dimethylthiotoluenediamine | 214.0 | 1.208 | - | - |
Catalysts
Polyurethane catalysts can be classified into two broad categories, amine compounds and organometallic complexes. They can be further classified as to their specificity, balance, and relative power or efficiency. Traditional amine catalysts have been tertiary amines such as triethylenediamine (TEDA, also known as 1,4-diazabicyclooctane or DABCO), dimethylcyclohexylamine (DMCHA), and dimethylethanolamine (DMEA). Tertiary amine catalysts are selected based on whether they drive the urethane (polyol+isocyanate, or gel) reaction, the urea (water+isocyanate, or blow) reaction, or the isocyanate trimerization reaction. Since most tertiary amine catalysts will drive all three reactions to some extent, they are also selected based on how much they favor one reaction over another. For example, tetramethylbutanediamine (TMBDA) preferentially drives the gel reaction over the blow reaction. On the other hand, both pentamethyldipropylenetriamine and N-(3-dimethylaminopropyl)-N,N-diisopropanolamine balance the blow and gel reactions, although the former is more potent than the later on a weight basis. 1,3,5-(tris(3-dimethylamino)propyl)-hexahydro-s-triazine is a trimerization catalyst that also strongly drives the blow reaction. Molecular structure gives some clue to the strength and selectivity of the catalyst. Blow catalysts generally have an ether linkage two carbons away from a tertiary nitrogen. Examples include bis-(2-dimethylaminoethyl)ether and N-ethylmorpholine. Strong gel catalysts contain alkyl-substituted nitrogens, such as triethylamine (TEA), 1,8-diazabicycloundecene-7 (DBU), and pentamethyldiethylenetriamine (PMDETA). Weaker gel catalysts contain ring-substituted nitrogens, such as benzyldimethylamine (BDMA). Trimerization catalysts contain the triazine structure, or are quaternary ammonium salts. Two trends have emerged since the late 1980s. The requirement to fill large, complex tooling with increasing production rates has led to the use of blocked catalysts to delay front end reactivity while maintaining back end cure. In the United States, acid- and quaternary ammonium salt-blocked TEDA and bis-(2-dimethylaminoethyl)ether are common blocked catalysts used in molded flexible foam and microcellular integral skin foam applications. Increasing aesthetic and environmental awareness has led to the use of non-fugitive catalysts for vehicle interior and furnishing applications in order to reduce odor, fogging, and the staining of vinyl coverings. Catalysts that contain a hydroxyl group or an active amino hydrogen, such as N,N,N'-trimethyl-N'-hydroxyethyl-bis(aminoethyl)ether and N'-(3-dimethylamino)propyl)-N,N-dimethyl-1,3-propanediamine that react into the polymer matrix can replace traditional catalysts in these applications.
Organometallic compounds based on mercury, lead, tin, bismuth, and zinc are used as polyurethane catalysts. Mercury carboxylates, such as phenylmercuric neodeconate, are particularly effective catalysts for polyurethane elastomer, coating and sealant applications, since they are very highly selective towards the polyol+isocyanate reaction. Mercury catalysts can be used at low levels to give systems a long pot life while still giving excellent back-end cure. Lead catalysts are used in highly reactive rigid spray foam insulation applications, since they maintain their potency in low-temperature and high-humidity conditions. Due to their toxicity and the necessity to dispose of mercury and lead catalysts and catalyzed material as hazardous waste in the United States, formulators have been searching for suitable replacements. Since the 1990s, bismuth and zinc carboxylates have been used as alternatives but have short comings of their own. In elastomer applications, long pot life systems do not build green strength as fast as mercury catalyzed systems. In spray foam applications, bismuth and zinc do not drive the front end fast enough in cold weather conditions and must be otherwise augmented to replace lead. Alkyl tin carboxylates, oxides and mercaptides oxides are used in all types of polyurethane applications. For example, dibutyltin dilaurate is a standard catalyst for polyurethane adhesives and sealants, dioctyltin mercaptide is used in microcellular elastomer applications, and dibutyltin oxide is used in polyurethane paint and coating applications. Tin mercaptides are used in formulations that contain water, as tin carboxylates are susceptible to degradation from hydrolysis.
Surfactants
Surfactants are used to modify the characteristics of both foam and non-foam polyurethane polymers. They take the form of polydimethylsiloxane-polyoxyalkylene block copolymers, silicone oils, nonylphenol ethoxylates, and other organic compounds. In foams, they are used to emulsify the liquid components, regulate cell size, and stabilize the cell structure to prevent collapse and sub-surface voids. In non-foam applications they are used as air release and anti-foaming agents, as wetting agents, and are used to eliminate surface defects such as pin holes, orange peel, and sink marks.
Production
The main polyurethane producing reaction is between a diisocyanate (aromatic and aliphatic types are available) and a polyol, typically a polypropylene glycol or polyester polyol, in the presence of catalysts and materials for controlling the cell structure, (surfactants) in the case of foams. Polyurethane can be made in a variety of densities and hardnesses by varying the type of monomer(s) used and adding other substances to modify their characteristics, notably density, or enhance their performance. Other additives can be used to improve the fire performance, stability in difficult chemical environments and other properties of the polyurethane products.
Though the properties of the polyurethane are determined mainly by the choice of polyol, the diisocyanate exerts some influence, and must be suited to the application. The cure rate is influenced by the functional group reactivity and the number of functional isocyanate groups. The mechanical properties are influenced by the functionality and the molecular shape. The choice of diisocyanate also affects the stability of the polyurethane upon exposure to light. Polyurethanes made with aromatic diisocyanates yellow with exposure to light, whereas those made with aliphatic diisocyanates are stable.
Softer, elastic, and more flexible polyurethanes result when linear difunctional polyethylene glycol segments, commonly called polyether polyols, are used to create the urethane links. This strategy is used to make spandex elastomeric fibers and soft rubber parts, as well as foam rubber. More rigid products result if polyfunctional polyols are used, as these create a three-dimensional cross-linked structure which, again, can be in the form of a low-density foam.
An even more rigid foam can be made with the use of specialty trimerization catalysts which create cyclic structures within the foam matrix, giving a harder, more thermally stable structure, designated as polyisocyanurate foams. Such properties are desired in rigid foam products used in the construction sector.
Careful control of viscoelastic properties — by modifying the catalysts and polyols used —can lead to memory foam, which is much softer at skin temperature than at room temperature.
There are then two main foam variants: one in which most of the foam bubbles (cells) remain closed, and the gas(es) remains trapped, the other being systems which have mostly open cells, resulting after a critical stage in the foam-making process (if cells did not form, or became open too soon, foam would not be created). This is a vitally important process: if the flexible foams have closed cells, their softness is severely compromised, they become pneumatic in feel, rather than soft; so, generally speaking, flexible foams are required to be open-celled.
The opposite is the case with most rigid foams. Here, retention of the cell gas is desired since this gas (especially the fluorocarbons referred to above) gives the foams their key characteristic: high thermal insulation performance.
A third foam variant, called microcellular foam, yields the tough elastomeric materials typically experienced in the coverings of car steering wheels and other interior automotive components.
Health and safety
Fully reacted polyurethane polymer, CAS # 9009-54-5 (CAS registry number), is chemically inert. In the United States, no exposure limits have been established by OSHA (Occupational Safety and Health Administration) or ACGIH (American Conference of Governmental Industrial Hygienists). It is not regulated by OSHA for carcinogenicity. Polyurethane polymer is a combustible solid and will ignite if exposed to an open flame for a sufficient period of time. Decomposition products include carbon monoxide, oxides of nitrogen, and hydrogen cyanide. Firefighters should wear self-contained breathing apparatus in enclosed areas. Polyurethane polymer dust can cause mechanical irritation to the eyes and lungs. Proper hygiene controls and personal protective equipment (PPE), such as gloves, dust masks, respirators, mechanical ventilation, and protective clothing and eye wear should be used.
Liquid resin blends and isocyanates may contain hazardous or regulated components. They should be handled in accordance with manufacturer recommendations found on product labels, and in MSDS (Material Safety Data Sheet) and product technical literature. Isocyanates are known skin and respiratory sensitizers, and proper engineering controls should be in place to prevent exposure to isocyanate liquid and vapor.
In the United States, additional health and safety information can be found through organizations such as the Polyurethane Manufacturers Association (PMA) and the Center for the Polyurethanes Industry (CPI), as well as from polyurethane system and raw material manufacturers. Regulatory information can be found in the Code of Federal Regulations Title 21 (Food and Drugs) and Title 40 (Protection of the Environment).
Uses
| characteristics of polyurethane materials |

|
Polyurethane products have many uses. Over three quarters of the global consumption of polyurethane products is in the form of foams, with flexible and rigid types being roughly equal in market size. In both cases, the foam is usually behind other materials: flexible foams are behind upholstery fabrics in commercial and domestic furniture; rigid foams are inside the metal and plastic walls of most refrigerators and freezers, or behind paper, metals and other surface materials in the case of thermal insulation panels in the construction sector. Its use in garments is growing: for example, in lining the cups of brassieres. Polyurethane is also used for moldings which include door frames, columns, balusters, window headers, pediments, medallions and rosettes.
The precursors of expanding polyurethane foam are available in many forms, for use in insulation, sound deadening, flotation, industrial coatings, packing material, and even cast-in-place upholstery padding. Since they adhere to most surfaces and automatically fill voids, they have become quite popular in these applications.
The following table shows how polyurethanes are used (US data from 2004):.
| Application | Amount of polyurethane used
(millions of pounds) |
Percentage of total |
|---|---|---|
| Building & Construction | 1,298 | 23.8% |
| Transportation | 1,298 | 23.8% |
| Furniture & Bedding | 1,127 | 20.7% |
| Appliances | 278 | 5.1% |
| Packaging | 251 | 4.6% |
| Textiles, Fibers & Apparel | 181 | 3.3% |
| Machinery & Foundry | 178 | 3.3% |
| Electronics | 75 | 1.4% |
| Footwear | 39 | 0.7% |
| Other uses | 558 | 10.2% |
| Total | 5,444 | 100.0% |
Varnish
Polyurethane materials are commonly formulated as paints and varnishes for finishing coats to protect or seal wood. This use results in a hard, abrasion-resistant, and durable coating that is popular for hardwood floors, but considered by some to be difficult or unsuitable for finishing furniture or other detailed pieces. Relative to oil or shellac varnishes, polyurethane varnish forms a harder film which tends to de-laminate if subjected to heat or shock, fracturing the film and leaving white patches. This tendency increases when it is applied over softer woods like pine. This is also in part due to polyurethane's lesser penetration into the wood. Various priming techniques are employed to overcome this problem, including the use of certain oil varnishes, specified "dewaxed" shellac, clear penetrating epoxy, or "oil-modified" polyurethane designed for the purpose. Polyurethane varnish may also lack the "hand-rubbed" lustre of drying oils such as linseed or tung oil; in contrast, however, it is capable of a much faster and higher "build" of film, accomplishing in two coats what may require multiple applications of oil. Polyurethane may also be applied over a straight oil finish, but because of the relatively slow curing time of oils, the presence of volatile byproducts of curing, and the need for extended exposure of the oil to oxygen, care must be taken that the oils are sufficiently cured to accept the polyurethane.
Unlike drying oils and alkyds which cure, after evaporation of the solvent, upon reaction with oxygen from the air, polyurethane coatings cure after evaporation of the solvent by a variety of reactions of chemicals within the original mix, or by reaction with moisture from the air. Certain products are "hybrids" and combine different aspects of their parent components. "Oil-modified" polyurethanes, whether water-borne or solvent-borne, are currently the most widely used wood floor finishes.
Exterior use of polyurethane varnish may be problematic due to its susceptibility to deterioration through ultra-violet light exposure. It must be noted, however, that all clear or transluscent varnishes, and indeed all film-polymer coatings (i.e.paint, stain, epoxy, synthetic plastic, etc.) are susceptible to this damage in varying degrees. Pigments in paints and stains protect against UV damage, while UV-absorbers are added to polyurethane and other varnishes (in particular "spar" varnish) to work against UV damage. Polyurethanes are typically the most resistant to water exposure, high humidity, temperature extremes, and fungus or mildew, which also adversely affect varnish and paint performance.
Wheels
Polyurethane is also used in making solid tires. Modern roller blading and skateboarding became economical only with the introduction of tough, abrasion-resistant polyurethane parts. Other constructions have been developed for pneumatic tires, and microcellular foam variants are widely used in tires on wheelchairs, bicycles and other such uses. These latter foam types are also widely encountered in car steering wheels and other interior and exterior automotive parts, including bumpers and fenders.
Furniture
Polyurethane is also used in furniture manufacture for casting soft edges around table tops and panel that are stylish, very durable and prevent injury. These are used in school tables, hospital and bank furniture as well as shop counters and displays.
Much of the foam used in chairs (including beanbag chairs), couches, mattresses is polyurethane foam. This type of foam is made by mixing polyols, diisocyanates, catalysts, blowing agents and other additives and allowing the resulting foam to rise freely. This can be done in a batch process where relatively small blocks of foam are made in an open-topped mold, or continuously where the components are poured onto an inclined moving belt. The foam is then cut to the desired shape and size for use in making furniture. Safety concerns about the flammability of polyurethane foam, particularly in upholstered furniture, sometimes requires the addition of flame retardants to this foam.
Polyurethane is in other countries like The Netherlands used for flooring in houses, offices and musea. Polyurethane is used on the bottom of some mouse pads.
Automobile seats
Flexible and semi-flexible polyurethane foams are used extensively for interior components of automobiles, in seats, headrests, armrests, roof liners and instrument panels.


Polyurethanes are used to make automobile seats in a remarkable manner. The seat manufacturer has a mold for each seat model. The mold is a closeable "clamshell" sort of structure that will allow quick casting of the seat cushion, so-called molded flexible foam, which is then upholstered after removal from the mold.
It is possible to combine these two steps, so-called in-situ, foam-in-fabric or direct moulding. A complete, fully-assembled seat cover is placed in the mold and held in place by vacuum drawn through small holes in the mold. Sometimes a thin pliable plastic film backing on the fabric is used to help the vacuum work more effectively. The metal seat frame is placed into the mold and the mold closed. At this point the mold contains what could be visualized as a "hollow seat", a seat fabric held in the correct position by the vacuum manifold and containing a hollow space with the metal frame in place.
Polyurethane chemicals are injected by a mixing head into the mold cavity. Then the mold is held at a preset reaction temperature until the chemical mixture has foamed, filled the mold, and formed a stable soft foam. The time required is about two to three minutes, depending on the size of the seat and the precise formulation and operating conditions. Then the mold is usually opened slightly for a minute or two for an additional cure time, before the fully upholstered seat is removed.
Houses, sculptures, and decorations
The walls and ceiling (not just the insulation) of the futuristic Xanadu House were built out of polyurethane foam. Domed ceilings and other odd shapes are easier to make with foam than with wood. Foam was used to build oddly-shaped buildings, statues, and decorations in the Seuss Landing section of the Islands of Adventure theme park. Speciality rigid foam manufactures sell foam that replace wood in carved sign and 3D topography industries .
Watercraft
Some surfboards are made with a solid polyurethane core. A rigid foam blank is molded, shaped to specification, then covered with fiberglass cloth and polyester resin.
The hull of the Boston Whaler motorboat is polyurethane foam sandwiched in a fiberglass skin. The foam provides strength, buoyancy, and sound deadening.
Construction sealants and firestopping


Polyurethane sealants are available in 1, 2 and even 3 part systems, either in cartridge, bucket or drum format. Polyurethane sealants are also sold for firestopping applications. Obviously, the sealant by itself provides no serious hindrance to fire, as its hydrocarbon bonds readily support combustion. However, when backed by inorganic insulation, such as rockwool or ceramic fibres, it can act as an effective seal to thwart smoke and hose-stream passage, particularly in inorganic joints. It is, however, advisable to avoid direct contact with metallic penetrants and through-penetrating cables, as the heat carried by the penetrants may jeopardise the sealant. This, however, requires a lot of vigilance. In concrete to concrete, or concrete to masonry joints, however, that are free of mechanical or electrical penetrants, it works well and dependably. As with all passive fire protection products and systems, the key to code compliance is demonstrable bounding.
Tennis Grips
Polyurethane has been used to make several Tennis Overgrips such as Yonex Supergrap, Wilson Pro Overgrip and many other grips. These grips are highly stretchable to ensure the grip wraps neatly around the racquet's handle.
Electronic Components
Often electronic components are protected from environmental influence and mechanical shock by enclosing them in polyurethane. Typically polyurethanes are selected for the excellent abrasion resistances, good electrical properties, excellent adhesion, impact strength,and low temperature flexibility. The disadvantage of polyurethanes is the limited upper service temperature (typically 250 °F (121 °C)). In production the electronic manufacture would purchase a two part urethane (resin and catalyst) that would be mixed and poured onto the circuit assembly (see Resin casting). In most cases, the final circuit board assembly would be unrepairable after the urethane has cured. Because of its physical properties and low cost, polyurethane encapsulation (potting) is a popular option in the automotive manufacturing sector for automotive circuits and sensors.
Adhesives
Polyurethane is used as an adhesive, especially as a woodworking glue. Its main advantage over more traditional wood glues is its water resistance. It was introduced in the general North American market in the 1990s as Gorilla Glue and Excel, but has been used much longer in Europe.
On the way to a new and better glue for bookbinders, a new adhesive system was introduced for the first time in 1985. The base for this system is polyether or polyester, whereas polyurethane (PUR) is used as prepolymer. Its special feature is the coagulation at room temperature and the reacting to moisture.
1st Generation (1988 at the drupa)
- Low starting solidity
- High viscosity
- Cure time of more than 3 days
2nd Generation (1996 at the drupa)
- Low starting solidity
- High viscosity
- Cure time of less than 3 days
3rd Generation (2000 at the drupa)
- Good starting solidity
- Low viscosity
- Cure time between 6 and 16 hours
4th Generation (present)
- Good starting solidity
- Very low viscosity
- Cure reached within a few seconds due to dual-core systems
Advantages of polyurethane glue in the bookbinding industry: PUR is real wonder compared to hotmelt and cold glue. Because of the missing moisture in the glue, papers with wrong grain direction can be processed without problems. Even printed and supercalandered paper can be bound without problems. It is the most economical glue with an application thickness of theoretical 0.01 mm. But in reality it is not possible to apply less than 0.03 mm. The PUR glue is very weather-proof and stable at temperatures from -40 °C to 100 °C.
Watch Band Wrapping
Polyurethane is used as a black wrapping for timepiece bracelets over the main material which is generally stainless steel. It is used for comfort, style, and durability.
See also
- Firestop
- Silicone
- Passive fire protection
- Bounding
- Penetrant
- Gorilla Glue
- Great Stuff sprayable foam
References
- see German Patent 728.981 (1937) I.G. Farben
- ^ Polyurethanes: A Class of Modern Versatile Materials Raymond B. Seymour George B. Kauffman J. Chem. Ed. 69, 909 1992
- Feske, Bert (October 2004). The Use of Saytex RB-9130/9170 Low Viscosity Brominated Flame Retardant Polyols in HFC-245fa and High Water Formulations. Las Vegas, NV: Alliance for the Polyurethane Industry Technical Conference.
{{cite conference}}:|access-date=requires|url=(help); Cite has empty unknown parameters:|booktitle=and|coauthors=(help); line feed character in|title=at position 57 (help) - Niemeyer, Timothy (September, 2006). A Further Examination of Soy-Based Polyols in Polyurethane Systems. Salt Lake City, UT: Alliance for the Polyurethane Industry Technical Conference.
{{cite conference}}:|access-date=requires|url=(help); Check date values in:|date=(help); Cite has empty unknown parameter:|booktitle=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - "New Twist on Green: 2008 Ford Mustang Seats Will Be Soy-Based Foam". Edmunds inside line. July 12, 2007. Retrieved 2007-10-02.
- Gum, Wilson (1992). Reaction Polymers. New York: Oxford University Press. ISBN 0-19-520933-8.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Harrington, Ron (1991). Flexible Polyurethane Foams. Midland: The Dow Chemical Company.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Oertel, Gunter (1985). Polyurethane Handbook. New York: Macmillen Publishing Co., Inc. ISBN 0-02-948920-2.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - Ulrich, Henri (1996). Chemistry and Technology of Isocyanates. New York: John Wiley & Sons, Inc. ISBN 0-471-96371-2.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - Woods, George (1990). The ICI Polyurethanes Book. New York: John Wiley & Sons, Inc. ISBN 0-471-92658-2.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - Kaushiva, Byran D. (August 15, 1999). "Structure-Property Relationships of Flexible Polyurethane Foams". PhD Thesis. Virginia Polytechnic Institute.
{{cite journal}}: Cite journal requires|journal=(help) - "Technical data sheet from Dow Chemical". Retrieved 2007-09-15.
- Oertel, Gunter (1985). Polyurethane Handbook. New York: Macmillen Publishing Co., Inc. ISBN 0-02-948920-2.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - Blackwell, J. (1981). "The Structure of the Hard Segments in MDI/diol/PTMA Polyurethane Elastomers". Washington, D.C.: American Chemical Society. 0097-6156/81/0172-0179.
{{cite journal}}: Cite journal requires|journal=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - Blackwell, John (1979). "Structure of the hard segments in polyurethane elastomers". IPC Business Press. 0032-3861/79/010013-05.
{{cite journal}}: Cite journal requires|journal=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - Grillo, D.J. (1992). "Physical Properties of Polyurethanes from Polyesters and Other Polyols". Polyurethanes '92 Conference Proceedings. New Orleans, LA: The Society of the Plastics Industry, Inc.
{{cite conference}}:|access-date=requires|url=(help); Unknown parameter|booktitle=ignored (|book-title=suggested) (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - Musselman, S.G. (1998). "Structure Versus Performance Properties of Cast Elastomers". Polyurethanes '98 Conference Proceedings. Dallas, TX: The Society of the Plastics Industry, Inc.
{{cite conference}}:|access-date=requires|url=(help); Unknown parameter|booktitle=ignored (|book-title=suggested) (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - Gum, Wilson (1992). Reaction Polymers. New York: Oxford University Press. ISBN 0-19-520933-8.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) -
"A Guide To Glycols", 117-00991-92HYC, The Dow Chemical Company, 1992
{{citation}}:|access-date=requires|url=(help);|format=requires|url=(help) - "Jeffcat Amine Catalysts for the Polyurethane Industry" (pdf). 2006. Retrieved 2007-10-23.
- "Building quality with Air Products trimerisation catalysts" (pdf). 2003. Retrieved 2007-10-23.
- "FOMREZ Specialty Tin Catalysts for Polyurethane Applications", 120-074-10, Crompton Corporation, 2001-01
{{citation}}:|access-date=requires|url=(help);|format=requires|url=(help); Check date values in:|date=(help) - "FOMREZ Specialty Tin Catalysts for Polyurethane Applications (leaflet insert)", 120-075-10, Crompton Corporation, 2001-01
{{citation}}:|access-date=requires|url=(help);|format=requires|url=(help); Check date values in:|date=(help) - Randall, David (2002). The Polyurethanes Book. New York: Wiley. ISBN 0-470-85041-8.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - "The Socio-Economic Impact of Polyurethanes in the United States from the American Chemistry Council" (PDF). The Polyurethanes Recycle and Recovery Council (PURRC), a committee of the Center for the Polyurethanes Industry. February 2004. Retrieved 2007-09-28.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help); External link in|publisher= - See:
- http://de.wikipedia.org/Klebstoff
External links
- Center for the Polyurethanes Industry : Provides information for EH&S issues related to polyurethanes

