| Revision as of 17:50, 11 August 2020 editKaldari (talk | contribs)Autopatrolled, Extended confirmed users, File movers, Pending changes reviewers, Rollbackers68,434 edits fixing edition param← Previous edit | Revision as of 15:40, 21 August 2020 edit undoBoghog (talk | contribs)Autopatrolled, Extended confirmed users, IP block exemptions, New page reviewers, Pending changes reviewers, Rollbackers, Template editors137,621 edits consistent citation formattingNext edit → | ||
| Line 22: | Line 22: | ||
| }} | }} | ||
| ] | ] | ||
| '''Typical antipsychotics''' (also known as '''first generation antipsychotics''', or '''FGAs''') are a class of ] drugs first developed in the 1950s and used to treat ] (in particular, ]). Typical antipsychotics may also be used for the treatment of acute mania, agitation, and other conditions. The first typical antipsychotics to come into medical use were the ]s, namely ] which was discovered ].<ref>{{cite journal | |
'''Typical antipsychotics''' (also known as '''first generation antipsychotics''', or '''FGAs''') are a class of ] drugs first developed in the 1950s and used to treat ] (in particular, ]). Typical antipsychotics may also be used for the treatment of acute mania, agitation, and other conditions. The first typical antipsychotics to come into medical use were the ]s, namely ] which was discovered ].<ref>{{cite journal | vauthors = Shen WW | title = A history of antipsychotic drug development | journal = Comprehensive Psychiatry | volume = 40 | issue = 6 | pages = 407–14 | year = 1999 | pmid = 10579370 | doi = 10.1016/s0010-440x(99)90082-2 }}</ref> Another prominent grouping of antipsychotics are the ], an example of which would be ]. The newer, second-generation antipsychotics, also known as ], have largely supplanted the use of typical antipsychotics as first-line agents due to the higher risk of movement disorders in the latter. | ||
| Both generations of medication tend to block receptors in the brain's ], but atypicals at the time of marketing were claimed to differ from typical antipsychotics in that they are less likely to cause ] (EPS), which include unsteady ]-type movements, ], and other involuntary movements (e.g. ], which can persist after stopping the medication).<ref>{{cite journal | |
Both generations of medication tend to block receptors in the brain's ], but atypicals at the time of marketing were claimed to differ from typical antipsychotics in that they are less likely to cause ] (EPS), which include unsteady ]-type movements, ], and other involuntary movements (e.g. ], which can persist after stopping the medication).<ref>{{cite journal | vauthors = | title = A roadmap to key pharmacologic principles in using antipsychotics | journal = Primary Care Companion to the Journal of Clinical Psychiatry | volume = 9 | issue = 6 | pages = 444–54 | year = 2007 | pmid = 18185824 | pmc = 2139919 | doi = 10.4088/PCC.v09n0607 }}</ref> More recent research has demonstrated the side effect profile of these drugs is similar to older drugs, causing the leading medical journal '']'' to write in its editorial "the time has come to abandon the terms first-generation and second-generation antipsychotics, as they do not merit this distinction."<ref>{{cite journal | vauthors = Tyrer P, Kendall T | title = The spurious advance of antipsychotic drug therapy | journal = Lancet | volume = 373 | issue = 9657 | pages = 4–5 | date = January 2009 | pmid = 19058841 | doi = 10.1016/S0140-6736(08)61765-1 }}</ref> While typical antipsychotics are more likely to cause EPS, atypicals are more likely to cause metabolic side effects, such as ] and increase the risk for ].<ref>{{cite web|url=http://www.rcpsych.ac.uk/healthadvice/treatmentswellbeing/antipsychoticmedication.aspx%7B%7Bfull%7D%7D|title=Not found|website=www.rcpsych.ac.uk|access-date=8 May 2018|url-status=live|archive-url=https://web.archive.org/web/20180508094647/https://www.rcpsych.ac.uk/healthadvice/treatmentswellbeing/antipsychoticmedication.aspx%7B%7Bfull%7D%7D|archive-date=8 May 2018}}</ref> | ||
| == Medical uses == | == Medical uses == | ||
| Line 32: | Line 32: | ||
| {{further|Phenothiazines}} | {{further|Phenothiazines}} | ||
| Side effects vary among the various agents in this class of medications, but common side effects include: dry mouth, ] stiffness, muscle cramping, ]s, ] and ] gain. EPS refers to a cluster of symptoms consisting of ], ], and ]. Anticholinergics such as ] and ] are commonly prescribed to treat the EPS. 4% of patients develop ] while on typical antipsychotics.<ref name="2870650PMID">{{cite journal | |
Side effects vary among the various agents in this class of medications, but common side effects include: dry mouth, ] stiffness, muscle cramping, ]s, ] and ] gain. EPS refers to a cluster of symptoms consisting of ], ], and ]. Anticholinergics such as ] and ] are commonly prescribed to treat the EPS. 4% of patients develop ] while on typical antipsychotics.<ref name="2870650PMID">{{cite journal | vauthors = Yassa R, Lal S | title = Prevalence of the rabbit syndrome | journal = The American Journal of Psychiatry | volume = 143 | issue = 5 | pages = 656–7 | date = May 1986 | pmid = 2870650 | doi = 10.1176/ajp.143.5.656 }}</ref> | ||
| There is a low risk of developing a serious condition called ] as a side effect of antipsychotics, including typical antipsychotics. The risk of developing tardive dyskinesia after chronic typical antipsychotic usage varies on several factors, such as age and gender, as well as the specific antipsychotic used. The commonly reported incidence of TD among younger patients is about 5% per year. Among older patients incidence rates as high as 20% per year have been reported. The average prevalence is approximately 30%.<ref>{{cite journal | |
There is a low risk of developing a serious condition called ] as a side effect of antipsychotics, including typical antipsychotics. The risk of developing tardive dyskinesia after chronic typical antipsychotic usage varies on several factors, such as age and gender, as well as the specific antipsychotic used. The commonly reported incidence of TD among younger patients is about 5% per year. Among older patients incidence rates as high as 20% per year have been reported. The average prevalence is approximately 30%.<ref>{{cite journal | vauthors = Llorca PM, Chereau I, Bayle FJ, Lancon C | title = Tardive dyskinesias and antipsychotics: a review | journal = European Psychiatry | volume = 17 | issue = 3 | pages = 129–38 | date = May 2002 | pmid = 12052573 | doi = 10.1016/S0924-9338(02)00647-8 }}</ref> There are few treatments that have consistently been shown to be effective for the treatment of tardive dyskinesia, though an ] inhibitor like ] may help.<ref name="FDA approves valben">{{cite web | author = Office of the Commissioner |title=FDA approves first drug to treat tardive dyskinesia |url=https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-treat-tardive-dyskinesia |website=FDA |access-date=18 June 2020 |language=en |date=24 March 2020}}</ref> The atypical antipsychotic ] has also been suggested as an alternative antipsychotic for patients experiencing tardive dyskinesia.<ref name="Pardis et al 2019">{{cite journal | vauthors = Pardis P, Remington G, Panda R, Lemez M, Agid O | title = Clozapine and tardive dyskinesia in patients with schizophrenia: A systematic review | journal = Journal of Psychopharmacology | volume = 33 | issue = 10 | pages = 1187–1198 | date = October 2019 | pmid = 31347436 | doi = 10.1177/0269881119862535 }}</ref> Tardive dyskinesia may reverse upon discontinuation of the offending agent or it may be irreversible, withdrawal may also make tardive dyskinesia more severe.<ref>{{cite web |url=https://medlineplus.gov/ency/article/000685.htm |title=Archived copy |access-date=2017-01-18 |url-status=live |archive-url=https://web.archive.org/web/20170131184044/https://medlineplus.gov/ency/article/000685.htm |archive-date=2017-01-31 }}{{full citation needed|date=September 2018}}</ref> | ||
| ], or NMS, is a rare, but potentially fatal side effect of antipsychotic treatment. NMS is characterized by fever, muscle rigidity, autonomic dysfunction, and altered mental status. Treatment includes discontinuation of the offending agent and supportive care. | ], or NMS, is a rare, but potentially fatal side effect of antipsychotic treatment. NMS is characterized by fever, muscle rigidity, autonomic dysfunction, and altered mental status. Treatment includes discontinuation of the offending agent and supportive care. | ||
| The role of typical antipsychotics has come into question recently as studies have suggested that typical antipsychotics may increase the risk of death in elderly patients. A retrospective cohort study from the ''New England Journal of Medicine'' on Dec. 1, 2005 showed an increase in risk of death with the use of typical antipsychotics that was on par with the increase shown with atypical antipsychotics.<ref>{{cite journal | |
The role of typical antipsychotics has come into question recently as studies have suggested that typical antipsychotics may increase the risk of death in elderly patients. A retrospective cohort study from the ''New England Journal of Medicine'' on Dec. 1, 2005 showed an increase in risk of death with the use of typical antipsychotics that was on par with the increase shown with atypical antipsychotics.<ref>{{cite journal | vauthors = Wang PS, Schneeweiss S, Avorn J, Fischer MA, Mogun H, Solomon DH, Brookhart MA | title = Risk of death in elderly users of conventional vs. atypical antipsychotic medications | journal = The New England Journal of Medicine | volume = 353 | issue = 22 | pages = 2335–41 | date = December 2005 | pmid = 16319382 | doi = 10.1056/NEJMoa052827 | url = https://semanticscholar.org/paper/90a99164f87020151bec186d7204f5569910a27a }}</ref> This has led some to question the common use of antipsychotics for the treatment of agitation in the elderly, particularly with the availability of alternatives such as mood stabilizing and antiepileptic drugs. | ||
| == Potency == | == Potency == | ||
| Line 53: | Line 53: | ||
| |} | |} | ||
| ] (Compazine, Buccastem, Stemetil) and ] (Orap) are less commonly used to treat psychotic states, and so are sometimes excluded from this classification.<ref name="isbn0-684-82737-9">{{cite book | |
] (Compazine, Buccastem, Stemetil) and ] (Orap) are less commonly used to treat psychotic states, and so are sometimes excluded from this classification.<ref name="isbn0-684-82737-9">{{cite book | last = Gitlin | first = Michael J. | name-list-format = vanc |title=The psychotherapist's guide to psychopharmacology |publisher=Free Press |location=New York |year=1996 |page=392 |isbn=0-684-82737-9 |oclc= |doi= |access-date=}}</ref> | ||
| A related concept to D2 potency is the concept of "chlorpromazine equivalence", which provides a measure of the relative effectiveness of antipsychotics.<ref name="pmid12823080">{{cite journal | |
A related concept to D2 potency is the concept of "chlorpromazine equivalence", which provides a measure of the relative effectiveness of antipsychotics.<ref name="pmid12823080">{{cite journal | vauthors = Woods SW | title = Chlorpromazine equivalent doses for the newer atypical antipsychotics | journal = The Journal of Clinical Psychiatry | volume = 64 | issue = 6 | pages = 663–7 | date = June 2003 | pmid = 12823080 | doi = 10.4088/JCP.v64n0607 }}</ref><ref name="pmid14624195">{{cite journal | vauthors = Rijcken CA, Monster TB, Brouwers JR, de Jong-van den Berg LT | title = Chlorpromazine equivalents versus defined daily doses: how to compare antipsychotic drug doses? | journal = Journal of Clinical Psychopharmacology | volume = 23 | issue = 6 | pages = 657–9 | date = December 2003 | pmid = 14624195 | doi = 10.1097/01.jcp.0000096247.29231.3a }}</ref> The measure specifies the amount (mass) in milligrams of a given drug that must be administered in order to achieve desired effects equivalent to those of 100 milligrams of chlorpromazine.<ref name="Patel et al 2013">{{cite journal | vauthors = Patel MX, Arista IA, Taylor M, Barnes TR | title = How to compare doses of different antipsychotics: a systematic review of methods | journal = Schizophrenia Research | volume = 149 | issue = 1-3 | pages = 141–8 | date = September 2013 | pmid = 23845387 | doi = 10.1016/j.schres.2013.06.030 }}</ref> Another method is "defined daily dose" (DDD), which is the assumed average dose of an antipsychotic that an adult would receive during long-term treatment.<ref name="Patel et al 2013" /> DDD is primarily used for comparing the utilization of antipsychotics (e.g. in an insurance claim database), rather than comparing therapeutic effects between antipsychotics.<ref name="Patel et al 2013" /> Maximum dose methods are sometimes used to compare between antipsychotics as well.<ref name="Patel et al 2013" /> It is important to note that these methods do not generally account for differences between the tolerability (i.e. the risk of side effects) or the safety between medications.<ref name="Patel et al 2013" /> | ||
| For a list of typical antipsychotics organized by potency, see below: | For a list of typical antipsychotics organized by potency, see below: | ||
| Line 85: | Line 85: | ||
| * ] | * ] | ||
| Where: † indicates products that have since been discontinued.<ref>{{cite book|title=Martindale: The Complete Drug Reference|publisher=The Royal Pharmaceutical Society of Great Britain| |
Where: † indicates products that have since been discontinued.<ref>{{cite book|title=Martindale: The Complete Drug Reference|publisher=The Royal Pharmaceutical Society of Great Britain|access-date=2 November 2013|year=2013|url=http://www.medicinescomplete.com/mc/martindale/current/}}</ref> | ||
| == Long-acting injectables == | == Long-acting injectables == | ||
| Some typical antipsychotics are available in a long-acting injectable (LAI), or "]", formulation. The first LAI antipsychotics (often referred to as simply "LAIs") were the typical antipsychotics fluphenazine and haloperidol.<ref name="Kennedy 2012">{{cite journal | |
Some typical antipsychotics are available in a long-acting injectable (LAI), or "]", formulation. The first LAI antipsychotics (often referred to as simply "LAIs") were the typical antipsychotics fluphenazine and haloperidol.<ref name="Kennedy 2012">{{cite journal | vauthors = Kennedy WK |title=When and how to use long-acting injectable antipsychotics |journal=Current Psychiatry |date=2012|volume=11 |issue=8 |pages=40-43 |url=https://www.mdedge.com/psychiatry/article/64796/schizophrenia-other-psychotic-disorders/when-and-how-use-long-acting |language=en}}</ref> Both fluphenazile and haloperidol are formulated as ], referring to the attachment of a decanoic acid group to the antipsychotic molecule.<ref name="Kennedy 2012" /> These are then dissolved in an organic oil.<ref name="Kennedy 2012" /> Together, these modifications prevent the active medications from being released immediately upon injection, attaining a slow release of the active medications (note, though, that the fluphenazine decanoate product is unique for reaching peak fluphenazine blood levels within 24 hours after administration<ref name="Carpenter et al 2018">{{cite journal | vauthors = Carpenter J, Wong KK |title=Long-acting injectable antipsychotics: What to do about missed doses |journal=Current Psychiatry |date=2018|volume=17 |issue=7 |pages=10-12,14-19,56 |url=https://www.mdedge.com/psychiatry/article/168776/schizophrenia-other-psychotic-disorders/long-acting-injectable |language=en}}</ref>).<ref name="Kennedy 2012" /> Fluphenazine decanoate can be administered every 7 to 21 days (usually every 14 to 28 days)<ref name="Carpenter et al 2018" />, while haloperidol decanoate can be administered every 28 days, though some people will require more or less frequent injections.<ref name="Kennedy 2012" /> If a scheduled injection of either haloperidol decanoate or fluphenazine decanoate is missed, recommendations for administering make-up injectable dose(s) or providing antipsychotics to be taken by mouth vary by, e.g., how long ago the last injection was and how many previous injections the person has received (i.e., if ] of the medication have been reached or not).<ref name="Carpenter et al 2018" /> | ||
| Both of the typical antipsychotic LAIs are inexpensive in comparison to the atypical LAIs.<ref name="Kennedy 2012" /> Atypical LAIs are usually preferred over typical LAIs due to the differences in side effects between typical and atypical antipsychotics in general.<ref name="Carpenter et al 2018" /> | Both of the typical antipsychotic LAIs are inexpensive in comparison to the atypical LAIs.<ref name="Kennedy 2012" /> Atypical LAIs are usually preferred over typical LAIs due to the differences in side effects between typical and atypical antipsychotics in general.<ref name="Carpenter et al 2018" /> | ||
| Line 98: | Line 98: | ||
| ]) from the 1950s, reflecting the perceptions of psychosis, including the now-discredited perception of a tendency towards violence, from the time when antipsychotics were discovered<ref>The text reads: "When the patient lashes out against 'them' - THORAZINE (brand of chlorpromazine) quickly puts an end to his violent outburst. 'Thorazine' is especially effective when the psychotic episode is triggered by delusions or hallucinations. At the outset of treatment, Thorazine's combination of antipsychotic and sedative effects provides both emotional and physical calming. Assaultive or destructive behavior is rapidly controlled. As therapy continues, the initial sedative effect gradually disappears. But the antipsychotic effect continues, helping to dispel or modify delusions, hallucinations and confusion, while keeping the patient calm and approachable. SMITH KLINE AND FRENCH LABORATORIES leaders in psychopharmaceutical research."</ref>]] | ]) from the 1950s, reflecting the perceptions of psychosis, including the now-discredited perception of a tendency towards violence, from the time when antipsychotics were discovered<ref>The text reads: "When the patient lashes out against 'them' - THORAZINE (brand of chlorpromazine) quickly puts an end to his violent outburst. 'Thorazine' is especially effective when the psychotic episode is triggered by delusions or hallucinations. At the outset of treatment, Thorazine's combination of antipsychotic and sedative effects provides both emotional and physical calming. Assaultive or destructive behavior is rapidly controlled. As therapy continues, the initial sedative effect gradually disappears. But the antipsychotic effect continues, helping to dispel or modify delusions, hallucinations and confusion, while keeping the patient calm and approachable. SMITH KLINE AND FRENCH LABORATORIES leaders in psychopharmaceutical research."</ref>]] | ||
| The original antipsychotic drugs were happened upon largely by chance and then tested for their effectiveness. The first, ], was developed as a surgical ] after an initial report in 1952.<ref name="Schatzberg 2019" /> It was first used on psychiatric patients because of its powerful calming effect; at the time it was regarded as a non-permanent "pharmacological ]".<ref name=pieters>{{cite journal | vauthors = Pieters T, Majerus B | title = The introduction of chlorpromazine in Belgium and the Netherlands (1951-1968); tango between old and new treatment features | journal = Studies in History and Philosophy of Biological and Biomedical Sciences | volume = 42 | issue = 4 | pages = 443–52 | date = December 2011 | pmid = 22035718 | doi = 10.1016/j.shpsc.2011.05.003 | url = http://orbilu.uni.lu/handle/10993/2251 | |
The original antipsychotic drugs were happened upon largely by chance and then tested for their effectiveness. The first, ], was developed as a surgical ] after an initial report in 1952.<ref name="Schatzberg 2019" /> It was first used on psychiatric patients because of its powerful calming effect; at the time it was regarded as a non-permanent "pharmacological ]".<ref name=pieters>{{cite journal | vauthors = Pieters T, Majerus B | title = The introduction of chlorpromazine in Belgium and the Netherlands (1951-1968); tango between old and new treatment features | journal = Studies in History and Philosophy of Biological and Biomedical Sciences | volume = 42 | issue = 4 | pages = 443–52 | date = December 2011 | pmid = 22035718 | doi = 10.1016/j.shpsc.2011.05.003 | url = http://orbilu.uni.lu/handle/10993/2251 | url-status = live | archive-url = https://web.archive.org/web/20170709194021/http://orbilu.uni.lu/handle/10993/2251 | df = dmy-all | archive-date = 9 July 2017 }}</ref> | ||
| Until the 1970s there was considerable debate within psychiatry on the most appropriate term to use to describe the new drugs.<ref name="king">{{cite journal | vauthors = King C, Voruganti LN | title = What's in a name? The evolution of the nomenclature of antipsychotic drugs | journal = Journal of Psychiatry & Neuroscience | volume = 27 | issue = 3 | pages = 168–75 | date = May 2002 | pmid = 12066446 | pmc = 161646 | doi = }}</ref> In the late 1950s the most widely used term was "neuroleptic", followed by "major ]" and then "ataraxic".<ref name="king"/> The word ''neuroleptic'' was coined in 1955 by Delay and Deniker after their discovery (1952) of the antipsychotic effects of chlorpromazine.<ref name="king"/> It is derived from the {{lang-el|"]"}} (''neuron'', originally meaning "]" but today referring to the ]s) and "]" (''lambanō'', meaning "take hold of"). Thus, the word means ''taking hold of one's nerves''. It was often taken to refer also to common side effects such as reduced activity in general, as well as lethargy and impaired motor control. Although these effects are unpleasant and in some cases harmful, they were at one time, along with akathisia, considered a reliable sign that the drug was working.<ref name=pieters /> These terms are being abandoned in favor of "antipsychotic", which refers to the medication's desired effects.<ref name="king"/> | Until the 1970s there was considerable debate within psychiatry on the most appropriate term to use to describe the new drugs.<ref name="king">{{cite journal | vauthors = King C, Voruganti LN | title = What's in a name? The evolution of the nomenclature of antipsychotic drugs | journal = Journal of Psychiatry & Neuroscience | volume = 27 | issue = 3 | pages = 168–75 | date = May 2002 | pmid = 12066446 | pmc = 161646 | doi = }}</ref> In the late 1950s the most widely used term was "neuroleptic", followed by "major ]" and then "ataraxic".<ref name="king"/> The word ''neuroleptic'' was coined in 1955 by Delay and Deniker after their discovery (1952) of the antipsychotic effects of chlorpromazine.<ref name="king"/> It is derived from the {{lang-el|"]"}} (''neuron'', originally meaning "]" but today referring to the ]s) and "]" (''lambanō'', meaning "take hold of"). Thus, the word means ''taking hold of one's nerves''. It was often taken to refer also to common side effects such as reduced activity in general, as well as lethargy and impaired motor control. Although these effects are unpleasant and in some cases harmful, they were at one time, along with akathisia, considered a reliable sign that the drug was working.<ref name=pieters /> These terms are being abandoned in favor of "antipsychotic", which refers to the medication's desired effects.<ref name="king"/> | ||
Revision as of 15:40, 21 August 2020
| Typical antipsychotic | |
|---|---|
| Drug class | |
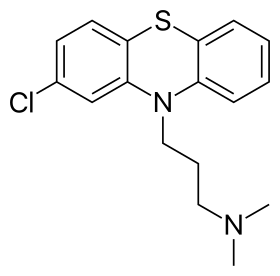 Skeletal formula of chlorpromazine, the first neuroleptic drug Skeletal formula of chlorpromazine, the first neuroleptic drug | |
| Synonyms | First generation antipsychotics, conventional antipsychotics, classical neuroleptics, traditional antipsychotics, major tranquilizers |
| Legal status | |
| In Wikidata | |

Typical antipsychotics (also known as first generation antipsychotics, or FGAs) are a class of antipsychotic drugs first developed in the 1950s and used to treat psychosis (in particular, schizophrenia). Typical antipsychotics may also be used for the treatment of acute mania, agitation, and other conditions. The first typical antipsychotics to come into medical use were the phenothiazines, namely chlorpromazine which was discovered serendipitously. Another prominent grouping of antipsychotics are the butyrophenones, an example of which would be haloperidol. The newer, second-generation antipsychotics, also known as atypical antipsychotics, have largely supplanted the use of typical antipsychotics as first-line agents due to the higher risk of movement disorders in the latter.
Both generations of medication tend to block receptors in the brain's dopamine pathways, but atypicals at the time of marketing were claimed to differ from typical antipsychotics in that they are less likely to cause extrapyramidal symptoms (EPS), which include unsteady Parkinson's disease-type movements, internal restlessness, and other involuntary movements (e.g. tardive dyskinesia, which can persist after stopping the medication). More recent research has demonstrated the side effect profile of these drugs is similar to older drugs, causing the leading medical journal The Lancet to write in its editorial "the time has come to abandon the terms first-generation and second-generation antipsychotics, as they do not merit this distinction." While typical antipsychotics are more likely to cause EPS, atypicals are more likely to cause metabolic side effects, such as weight gain and increase the risk for type II diabetes.
Medical uses
Typical antipsychotics block the dopamine 2 receptor (D2) receptor, causing an antipsychotic effect. It is thought that 60-80% of D2 receptors need to be occupied for antipsychotic effect. For reference, the typical antipsychotic haloperidol tends to block about 80% of D2 receptors at doses ranging from 2 to 5 mg per day. On the aggregate level, no typical antipsychotic is more effective than any other, though people will vary in which antipsychotic they prefer to take (based on individual differences in tolerability and effectiveness). Typical antipsychotics can be used to treat, e.g., schizophrenia or severe agitation. Haloperidol, due to the availability of a rapid-acting injectable formulation and decades of use, remains the most commonly used antipsychotic for treating severe agitation in the emergency department setting.
Adverse effects
Further information: PhenothiazinesSide effects vary among the various agents in this class of medications, but common side effects include: dry mouth, muscle stiffness, muscle cramping, tremors, EPS and weight gain. EPS refers to a cluster of symptoms consisting of akathisia, parkinsonism, and dystonia. Anticholinergics such as benztropine and diphenhydramine are commonly prescribed to treat the EPS. 4% of patients develop rabbit syndrome while on typical antipsychotics.
There is a low risk of developing a serious condition called tardive dyskinesia as a side effect of antipsychotics, including typical antipsychotics. The risk of developing tardive dyskinesia after chronic typical antipsychotic usage varies on several factors, such as age and gender, as well as the specific antipsychotic used. The commonly reported incidence of TD among younger patients is about 5% per year. Among older patients incidence rates as high as 20% per year have been reported. The average prevalence is approximately 30%. There are few treatments that have consistently been shown to be effective for the treatment of tardive dyskinesia, though an VMAT2 inhibitor like valbenazine may help. The atypical antipsychotic clozapine has also been suggested as an alternative antipsychotic for patients experiencing tardive dyskinesia. Tardive dyskinesia may reverse upon discontinuation of the offending agent or it may be irreversible, withdrawal may also make tardive dyskinesia more severe.
Neuroleptic malignant syndrome, or NMS, is a rare, but potentially fatal side effect of antipsychotic treatment. NMS is characterized by fever, muscle rigidity, autonomic dysfunction, and altered mental status. Treatment includes discontinuation of the offending agent and supportive care.
The role of typical antipsychotics has come into question recently as studies have suggested that typical antipsychotics may increase the risk of death in elderly patients. A retrospective cohort study from the New England Journal of Medicine on Dec. 1, 2005 showed an increase in risk of death with the use of typical antipsychotics that was on par with the increase shown with atypical antipsychotics. This has led some to question the common use of antipsychotics for the treatment of agitation in the elderly, particularly with the availability of alternatives such as mood stabilizing and antiepileptic drugs.
Potency
Traditional antipsychotics are classified as high-potency, mid-potency, or low-potency based on their potency for the D2 receptor:
| Potency | Examples | Adverse effect profile |
| high | fluphenazine and haloperidol | more extrapyramidal side effects (EPS) and less antihistaminic effects (e.g. sedation), alpha adrenergic antagonism (e.g. orthostatic hypotension), and anticholinergic effects (e.g. dry mouth) |
| middle | perphenazine and loxapine | intermediate D2 affinity, with more off-target effects than high-potency agents |
| low | chlorpromazine | less risk of EPS but more antihistaminic effects, alpha adrenergic antagonism, and anticholinergic effects |
Prochlorperazine (Compazine, Buccastem, Stemetil) and Pimozide (Orap) are less commonly used to treat psychotic states, and so are sometimes excluded from this classification.
A related concept to D2 potency is the concept of "chlorpromazine equivalence", which provides a measure of the relative effectiveness of antipsychotics. The measure specifies the amount (mass) in milligrams of a given drug that must be administered in order to achieve desired effects equivalent to those of 100 milligrams of chlorpromazine. Another method is "defined daily dose" (DDD), which is the assumed average dose of an antipsychotic that an adult would receive during long-term treatment. DDD is primarily used for comparing the utilization of antipsychotics (e.g. in an insurance claim database), rather than comparing therapeutic effects between antipsychotics. Maximum dose methods are sometimes used to compare between antipsychotics as well. It is important to note that these methods do not generally account for differences between the tolerability (i.e. the risk of side effects) or the safety between medications.
For a list of typical antipsychotics organized by potency, see below:
Low potency
- Chlorpromazine
- Chlorprothixene
- Levomepromazine
- Mesoridazine
- Periciazine
- Promazine
- Thioridazine† (withdrawn by brand-name manufacturer and most countries)
Medium potency
High potency
- Droperidol
- Flupentixol
- Fluphenazine
- Haloperidol
- Pimozide
- Prochlorperazine
- Thioproperazine
- Trifluoperazine
- Zuclopenthixol
Where: † indicates products that have since been discontinued.
Long-acting injectables
Some typical antipsychotics are available in a long-acting injectable (LAI), or "depot", formulation. The first LAI antipsychotics (often referred to as simply "LAIs") were the typical antipsychotics fluphenazine and haloperidol. Both fluphenazile and haloperidol are formulated as decanoates, referring to the attachment of a decanoic acid group to the antipsychotic molecule. These are then dissolved in an organic oil. Together, these modifications prevent the active medications from being released immediately upon injection, attaining a slow release of the active medications (note, though, that the fluphenazine decanoate product is unique for reaching peak fluphenazine blood levels within 24 hours after administration). Fluphenazine decanoate can be administered every 7 to 21 days (usually every 14 to 28 days), while haloperidol decanoate can be administered every 28 days, though some people will require more or less frequent injections. If a scheduled injection of either haloperidol decanoate or fluphenazine decanoate is missed, recommendations for administering make-up injectable dose(s) or providing antipsychotics to be taken by mouth vary by, e.g., how long ago the last injection was and how many previous injections the person has received (i.e., if steady state levels of the medication have been reached or not).
Both of the typical antipsychotic LAIs are inexpensive in comparison to the atypical LAIs. Atypical LAIs are usually preferred over typical LAIs due to the differences in side effects between typical and atypical antipsychotics in general.
| Medication | Brand name | Class | Vehicle | Dosage | Tmax | t1/2 single | t1/2 multiple | logP | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Aripiprazole lauroxil | Aristada | Atypical | Water | 441–1064 mg/4–8 weeks | 24–35 days | ? | 54–57 days | 7.9–10.0 | |
| Aripiprazole monohydrate | Abilify Maintena | Atypical | Water | 300–400 mg/4 weeks | 7 days | ? | 30–47 days | 4.9–5.2 | |
| Bromperidol decanoate | Impromen Decanoas | Typical | Sesame oil | 40–300 mg/4 weeks | 3–9 days | ? | 21–25 days | 7.9 | |
| Clopentixol decanoate | Sordinol Depot | Typical | Viscoleo | 50–600 mg/1–4 weeks | 4–7 days | ? | 19 days | 9.0 | |
| Flupentixol decanoate | Depixol | Typical | Viscoleo | 10–200 mg/2–4 weeks | 4–10 days | 8 days | 17 days | 7.2–9.2 | |
| Fluphenazine decanoate | Prolixin Decanoate | Typical | Sesame oil | 12.5–100 mg/2–5 weeks | 1–2 days | 1–10 days | 14–100 days | 7.2–9.0 | |
| Fluphenazine enanthate | Prolixin Enanthate | Typical | Sesame oil | 12.5–100 mg/1–4 weeks | 2–3 days | 4 days | ? | 6.4–7.4 | |
| Fluspirilene | Imap, Redeptin | Typical | Water | 2–12 mg/1 week | 1–8 days | 7 days | ? | 5.2–5.8 | |
| Haloperidol decanoate | Haldol Decanoate | Typical | Sesame oil | 20–400 mg/2–4 weeks | 3–9 days | 18–21 days | 7.2–7.9 | ||
| Olanzapine pamoate | Zyprexa Relprevv | Atypical | Water | 150–405 mg/2–4 weeks | 7 days | ? | 30 days | – | |
| Oxyprothepin decanoate | Meclopin | Typical | ? | ? | ? | ? | ? | 8.5–8.7 | |
| Paliperidone palmitate | Invega Sustenna | Atypical | Water | 39–819 mg/4–12 weeks | 13–33 days | 25–139 days | ? | 8.1–10.1 | |
| Perphenazine decanoate | Trilafon Dekanoat | Typical | Sesame oil | 50–200 mg/2–4 weeks | ? | ? | 27 days | 8.9 | |
| Perphenazine enanthate | Trilafon Enanthate | Typical | Sesame oil | 25–200 mg/2 weeks | 2–3 days | ? | 4–7 days | 6.4–7.2 | |
| Pipotiazine palmitate | Piportil Longum | Typical | Viscoleo | 25–400 mg/4 weeks | 9–10 days | ? | 14–21 days | 8.5–11.6 | |
| Pipotiazine undecylenate | Piportil Medium | Typical | Sesame oil | 100–200 mg/2 weeks | ? | ? | ? | 8.4 | |
| Risperidone | Risperdal Consta | Atypical | Microspheres | 12.5–75 mg/2 weeks | 21 days | ? | 3–6 days | – | |
| Zuclopentixol acetate | Clopixol Acuphase | Typical | Viscoleo | 50–200 mg/1–3 days | 1–2 days | 1–2 days | 4.7–4.9 | ||
| Zuclopentixol decanoate | Clopixol Depot | Typical | Viscoleo | 50–800 mg/2–4 weeks | 4–9 days | ? | 11–21 days | 7.5–9.0 | |
| Note: All by intramuscular injection. Footnotes: = Microcrystalline or nanocrystalline aqueous suspension. = Low-viscosity vegetable oil (specifically fractionated coconut oil with medium-chain triglycerides). = Predicted, from PubChem and DrugBank. Sources: Main: See template. | |||||||||
History
Main article: Antipsychotic § History
The original antipsychotic drugs were happened upon largely by chance and then tested for their effectiveness. The first, chlorpromazine, was developed as a surgical anesthetic after an initial report in 1952. It was first used on psychiatric patients because of its powerful calming effect; at the time it was regarded as a non-permanent "pharmacological lobotomy".
Until the 1970s there was considerable debate within psychiatry on the most appropriate term to use to describe the new drugs. In the late 1950s the most widely used term was "neuroleptic", followed by "major tranquilizer" and then "ataraxic". The word neuroleptic was coined in 1955 by Delay and Deniker after their discovery (1952) of the antipsychotic effects of chlorpromazine. It is derived from the Template:Lang-el (neuron, originally meaning "sinew" but today referring to the nerves) and "λαμβάνω" (lambanō, meaning "take hold of"). Thus, the word means taking hold of one's nerves. It was often taken to refer also to common side effects such as reduced activity in general, as well as lethargy and impaired motor control. Although these effects are unpleasant and in some cases harmful, they were at one time, along with akathisia, considered a reliable sign that the drug was working. These terms are being abandoned in favor of "antipsychotic", which refers to the medication's desired effects.
See also
References
- Shen WW (1999). "A history of antipsychotic drug development". Comprehensive Psychiatry. 40 (6): 407–14. doi:10.1016/s0010-440x(99)90082-2. PMID 10579370.
- "A roadmap to key pharmacologic principles in using antipsychotics". Primary Care Companion to the Journal of Clinical Psychiatry. 9 (6): 444–54. 2007. doi:10.4088/PCC.v09n0607. PMC 2139919. PMID 18185824.
- Tyrer P, Kendall T (January 2009). "The spurious advance of antipsychotic drug therapy". Lancet. 373 (9657): 4–5. doi:10.1016/S0140-6736(08)61765-1. PMID 19058841.
- "Not found". www.rcpsych.ac.uk. Archived from the original on 8 May 2018. Retrieved 8 May 2018.
- ^ Schatzberg's Manual of Clinical Psychopharmacology (Ninth ed.). American Psychiatric Association Publishing. 2019. ISBN 978-1-61537-230-0.
- Yassa R, Lal S (May 1986). "Prevalence of the rabbit syndrome". The American Journal of Psychiatry. 143 (5): 656–7. doi:10.1176/ajp.143.5.656. PMID 2870650.
- Llorca PM, Chereau I, Bayle FJ, Lancon C (May 2002). "Tardive dyskinesias and antipsychotics: a review". European Psychiatry. 17 (3): 129–38. doi:10.1016/S0924-9338(02)00647-8. PMID 12052573.
- Office of the Commissioner (24 March 2020). "FDA approves first drug to treat tardive dyskinesia". FDA. Retrieved 18 June 2020.
- Pardis P, Remington G, Panda R, Lemez M, Agid O (October 2019). "Clozapine and tardive dyskinesia in patients with schizophrenia: A systematic review". Journal of Psychopharmacology. 33 (10): 1187–1198. doi:10.1177/0269881119862535. PMID 31347436.
- "Archived copy". Archived from the original on 2017-01-31. Retrieved 2017-01-18.
{{cite web}}: CS1 maint: archived copy as title (link) - Wang PS, Schneeweiss S, Avorn J, Fischer MA, Mogun H, Solomon DH, Brookhart MA (December 2005). "Risk of death in elderly users of conventional vs. atypical antipsychotic medications". The New England Journal of Medicine. 353 (22): 2335–41. doi:10.1056/NEJMoa052827. PMID 16319382.
- Gitlin, Michael J. (1996). The psychotherapist's guide to psychopharmacology. New York: Free Press. p. 392. ISBN 0-684-82737-9.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - Woods SW (June 2003). "Chlorpromazine equivalent doses for the newer atypical antipsychotics". The Journal of Clinical Psychiatry. 64 (6): 663–7. doi:10.4088/JCP.v64n0607. PMID 12823080.
- Rijcken CA, Monster TB, Brouwers JR, de Jong-van den Berg LT (December 2003). "Chlorpromazine equivalents versus defined daily doses: how to compare antipsychotic drug doses?". Journal of Clinical Psychopharmacology. 23 (6): 657–9. doi:10.1097/01.jcp.0000096247.29231.3a. PMID 14624195.
- ^ Patel MX, Arista IA, Taylor M, Barnes TR (September 2013). "How to compare doses of different antipsychotics: a systematic review of methods". Schizophrenia Research. 149 (1–3): 141–8. doi:10.1016/j.schres.2013.06.030. PMID 23845387.
- Martindale: The Complete Drug Reference. The Royal Pharmaceutical Society of Great Britain. 2013. Retrieved 2 November 2013.
- ^ Kennedy WK (2012). "When and how to use long-acting injectable antipsychotics". Current Psychiatry. 11 (8): 40–43.
- ^ Carpenter J, Wong KK (2018). "Long-acting injectable antipsychotics: What to do about missed doses". Current Psychiatry. 17 (7): 10–12, 14–19, 56.
- Parent M, Toussaint C, Gilson H (1983). "Long-term treatment of chronic psychotics with bromperidol decanoate: clinical and pharmacokinetic evaluation". Current Therapeutic Research. 34 (1): 1–6.
- ^ Jørgensen A, Overø KF (1980). "Clopenthixol and flupenthixol depot preparations in outpatient schizophrenics. III. Serum levels". Acta Psychiatrica Scandinavica. Supplementum. 279: 41–54. doi:10.1111/j.1600-0447.1980.tb07082.x. PMID 6931472.
- ^ Reynolds JE (1993). "Anxiolytic sedatives, hypnotics and neuroleptics.". Martindale: The Extra Pharmacopoeia (30th ed.). London: Pharmaceutical Press. pp. 364–623.
- Ereshefsky L, Saklad SR, Jann MW, Davis CM, Richards A, Seidel DR (May 1984). "Future of depot neuroleptic therapy: pharmacokinetic and pharmacodynamic approaches". The Journal of Clinical Psychiatry. 45 (5 Pt 2): 50–9. PMID 6143748.
- ^ Curry SH, Whelpton R, de Schepper PJ, Vranckx S, Schiff AA (April 1979). "Kinetics of fluphenazine after fluphenazine dihydrochloride, enanthate and decanoate administration to man". British Journal of Clinical Pharmacology. 7 (4): 325–31. doi:10.1111/j.1365-2125.1979.tb00941.x. PMC 1429660. PMID 444352.
- Young D, Ereshefsky L, Saklad SR, Jann MW, Garcia N (1984). Explaining the pharmacokinetics of fluphenazine through computer simulations. (Abstract.). 19th Annual Midyear Clinical Meeting of the American Society of Hospital Pharmacists. Dallas, Texas.
- Janssen PA, Niemegeers CJ, Schellekens KH, Lenaerts FM, Verbruggen FJ, van Nueten JM, Marsboom RH, Hérin VV, Schaper WK (November 1970). "The pharmacology of fluspirilene (R 6218), a potent, long-acting and injectable neuroleptic drug". Arzneimittel-Forschung. 20 (11): 1689–98. PMID 4992598.
- Beresford R, Ward A (January 1987). "Haloperidol decanoate. A preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in psychosis". Drugs. 33 (1): 31–49. doi:10.2165/00003495-198733010-00002. PMID 3545764.
- Reyntigens AJ, Heykants JJ, Woestenborghs RJ, Gelders YG, Aerts TJ (1982). "Pharmacokinetics of haloperidol decanoate. A 2-year follow-up". International Pharmacopsychiatry. 17 (4): 238–46. doi:10.1159/000468580. PMID 7185768.
- Larsson M, Axelsson R, Forsman A (1984). "On the pharmacokinetics of perphenazine: a clinical study of perphenazine enanthate and decanoate". Current Therapeutic Research. 36 (6): 1071–88.
- The text reads: "When the patient lashes out against 'them' - THORAZINE (brand of chlorpromazine) quickly puts an end to his violent outburst. 'Thorazine' is especially effective when the psychotic episode is triggered by delusions or hallucinations. At the outset of treatment, Thorazine's combination of antipsychotic and sedative effects provides both emotional and physical calming. Assaultive or destructive behavior is rapidly controlled. As therapy continues, the initial sedative effect gradually disappears. But the antipsychotic effect continues, helping to dispel or modify delusions, hallucinations and confusion, while keeping the patient calm and approachable. SMITH KLINE AND FRENCH LABORATORIES leaders in psychopharmaceutical research."
- ^ Pieters T, Majerus B (December 2011). "The introduction of chlorpromazine in Belgium and the Netherlands (1951-1968); tango between old and new treatment features". Studies in History and Philosophy of Biological and Biomedical Sciences. 42 (4): 443–52. doi:10.1016/j.shpsc.2011.05.003. PMID 22035718. Archived from the original on 9 July 2017.
- ^ King C, Voruganti LN (May 2002). "What's in a name? The evolution of the nomenclature of antipsychotic drugs". Journal of Psychiatry & Neuroscience. 27 (3): 168–75. PMC 161646. PMID 12066446.