| Revision as of 13:07, 3 January 2007 editAgjchs (talk | contribs)104 edits added external link← Previous edit | Revision as of 14:04, 3 January 2007 edit undoJmax- (talk | contribs)910 editsm Reverted edits by Agjchs to last version by OhnoitsjamieNext edit → | ||
| Line 73: | Line 73: | ||
| * | * | ||
| * | * | ||
| * free PDF containing comprehensive charts | |||
| Lunbeck's official websites for citalopram under the trade name Cipramil: | Lunbeck's official websites for citalopram under the trade name Cipramil: | ||
Revision as of 14:04, 3 January 2007
Pharmaceutical compound | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 80% |
| Metabolism | hepatic (CYP3A4 & CYP2C19) |
| Elimination half-life | 35 hours |
| Excretion | Mostly as unmetabolized Citalopram, partly DCT and traces of DDCT |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.056.247 |
| Chemical and physical data | |
| Formula | C20H21FN2O |
| Molar mass | 324.392 g/mol g·mol |
Citalopram is an antidepressant drug used to treat depression associated with mood disorders. It is also used on occasion in the treatment of body dysmorphic disorder and anxiety.
Citalopram belongs to a class of drugs known as selective serotonin reuptake inhibitors (SSRIs). It is sold under the brand-names Celexa™ (U.S., Forest Laboratories, Inc.), Cipramil™ , Seropram™ (Europe and Australia), Celepram™ and Ciazil™ (Australia).
History
Citalopram was originally created by the pharmaceutical company Lundbeck and the patent for it expired in 2003, allowing other companies legally to produce generic versions.
Lundbeck has recently released an updated formulation called escitalopram oxalate (also known as Cipralex™ or Lexapro™), which is simply the S-enantiomer of the racemic citalopram (see below), and acquired a new patent for it.
Indications
Approved
Citalopram is primarily used to treat the symptoms of depression but can also be prescribed for social anxiety disorder, panic disorder or obsessive-compulsive disorder. Also prescribed in Huntington's Disease.
Unapproved/Off-label/Investigational
Citalopram has been found to significantly reduce the symptoms of diabetic neuropathy and premature ejaculation. There is also evidence that citalopram may be effective in the treatment of post-stroke pathological crying.
Side effects and drug interactions
Citalopram is safe and well-tolerated in the therapeutic dose range of 20 to 60 mg/day. Distinct from some other agents in its class, Citalopram exhibits linear pharmacokinetics and minimal drug interaction potential, making it a better choice for the elderly or comorbid patients.
Citalopram can have a number of adverse effects. In clinical trials, over 10% of patients reported fatigue, drowsiness, dry mouth, increased sweating (hyperhidrosis), trembling, headache, dizziness, sleep disturbances, cardiac arrythmia, blood pressure, nausea/vomiting, diarrhea, heightened anorgasmia in females, impotence and ejaculatory problems. In rare cases (around over 1% of cases), some allergic reactions, convulsions, mood changes, anxiety and confusion have been reported. Another uncommon side effect is bruxism (teeth grinding). Occasionally, panic attacks, thoughts of suicide or self-harm may occur or increase in the first few weeks, before the antidepressant effect starts.
Citalopram and other SSRIs have been shown to cause sexual side effects in some patients, both males and females. Although usually reversible, these sexual side effects can sometimes last for months, years or possibly indefinitely even after the drug has been completely withdrawn. This disorder is known as Post SSRI Sexual Dysfunction.
Citalopram is contraindicated in individuals taking MAOIs. It is considered relatively safe in overdose, although fatal cases of dosages 840 mg to 1960 mg have been reported.
Stereochemistry
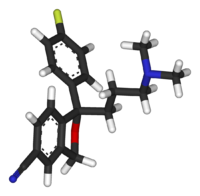
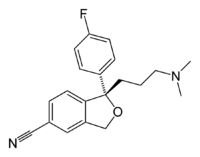
Citalopram has a stereocenter, to which a 4-fluorophenyl group and an N,N-dimethyl-3-aminopropyl group bind. Due to this chirality the molecule exists in (two) enantiomeric forms (mirror images). They are termed S-(+)-citalopram and R-(−)-citalopram.
 |
 |
 |
 |
| S-(+)-citalopram | R-(−)-citalopram |
Citalopram is sold as a racemic mixture, consisting of 50% R-(−)-citalopram and 50% S-(+)-citalopram. Only the S-(+) enantiomer has the desired antidepressant effect. Lundbeck now markets the S-(+) enantiomer, the generic name of which is escitalopram. Whereas citalopram is supplied as the hydrobromide, escitalopram is sold as the oxalate salt. The salt form makes these otherwise lipophilic compounds watersoluble.
External links
Pharmacological information and treatment study information:
- http://www.biopsychiatry.com/citalopram.html
- Celexa / Citalopram Fact Sheet
- FDA patient fact sheet on Citalopram hydrobromide
Lunbeck's official websites for citalopram under the trade name Cipramil:
Forest's official websites for citalopram under the trade name Celexa:
- Cipramil Patient Information Leaflet Cipramil Patient Information Leaflet
References
- "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- Sindrup SH, Bjerre U, Dejgaard A, Brosen K, Aaes-Jorgensen T, Gram LF. (1992). "The selective serotonin reuptake inhibitor citalopram relieves the symptoms of diabetic neuropathy". Clinical Pharmacology & Therapeutics. 52 (5): 547–552. PMID 1424428.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Atmaca M, Kuloglu M, Tezcan E, Semercioz A. (2002). "The efficacy of citalopram in the treatment of premature ejaculation: a placebo-controlled study". International Journal of Impotence Research. 14 (6): 502–505. PMID 12494286.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Andersen G., Vestergaard K., Riis JO. (1993). "Citalopram for post-stroke pathological crying". Lancet(British edition). 342 (8875): 837–839. PMID 8104273.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - http://www.biopsychiatry.com/citalopram.html
- http://www.mentalhealth.com/drug/p30-c04.html#Head_5
- Clayton A, Keller A, McGarvey EL. Burden of phase-specific sexual dysfunction with SSRIs. J Affect Disord 2006;91:27-32. PMID 16430968.
- http://www.mentalhealth.com/drug/p30-c04.html#Head_12
- Celexa.com