This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 01:46, 16 February 2012 (Updating {{chembox}} (changes to verified fields - added verified revid - updated '') per Chem/Drugbox validation (report errors or bugs)). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 01:46, 16 February 2012 by CheMoBot (talk | contribs) (Updating {{chembox}} (changes to verified fields - added verified revid - updated '') per Chem/Drugbox validation (report errors or bugs))(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) This article is about the chemical compound. For other uses, see KCL.
| |
| |
| Names | |
|---|---|
| Other names
Sylvite Muriate of potash | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.028.374 |
| E number | E508 (acidity regulators, ...) |
| KEGG | |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | KCl |
| Molar mass | 74.5513 g mol |
| Appearance | white crystalline solid |
| Odor | odorless |
| Density | 1.984 g/cm |
| Melting point | 770 °C |
| Boiling point | 1420 °C |
| Solubility in water | 281 g/L (0°C) 344 g/L (20°C) 567 g/L (100°C) |
| Solubility | soluble in glycerol, alkalies slightly soluble in alcohol, insoluble in ether |
| Acidity (pKa) | ~7 |
| Refractive index (nD) | 1.4902 (589 nm) |
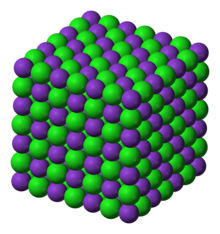
| Structure | |
| Crystal structure | face centered cubic |
| Thermochemistry | |
| Std molar entropy (S298) |
83 J·mol·K |
| Std enthalpy of formation (ΔfH298) |
−436 kJ·mol |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 2.6 g/kg (oral/rat), 0.142 g/kg (intravenous/rat) |
| Related compounds | |
| Other anions | Potassium fluoride Potassium bromide Potassium iodide |
| Other cations | Lithium chloride Sodium chloride Rubidium chloride Caesium chloride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
The chemical compound potassium chloride (KCl) is a metal halide salt composed of potassium and chlorine. In its pure state, it is odorless and has a white or colorless vitreous crystal appearance, with a crystal structure that cleaves easily in three directions. Potassium chloride crystals are face-centered cubic. Potassium chloride was historically known as "muriate of potash," this name is occasionally still encountered in association with its use as a fertilizer. Potash varies in color from pink or red to white depending on the mining and recovery process used. White potash, sometimes referred to as soluble potash, is usually higher in analysis and is used primarily for making liquid starter fertilizers. KCl is used in medicine, scientific applications, and food processing. It occurs naturally as the mineral sylvite and in combination with sodium chloride as sylvinite.
Chemical properties
In chemistry and physics, it is a very commonly used standard, for example as a calibration standard solution in measuring electrical conductivity of (ionic) solutions, since carefully prepared KCl solutions have well-reproducible and well-repeatable measurable properties.
| Solubility of KCl in various solvents (g KCl / 1 kg of solvent at 25°C) | |
|---|---|
| H2O | 360 |
| Liquid ammonia | 0.4 |
| Liquid sulfur dioxide | 0.41 |
| Methanol | 5.3 |
| Formic acid | 192 |
| Sulfolane | 0.04 |
| Acetonitrile | 0.024 |
| Acetone | 0.00091 |
| Formamide | 62 |
| Acetamide | 24.5 |
| Dimethylformamide | 0.17–0.5 |
Potassium chloride can react as a source of chloride ion. As with any other soluble ionic chloride, it will precipitate insoluble chloride salts when added to a solution of an appropriate metal ion:
Although potassium is more electropositive than sodium, KCl can be reduced to the metal by reaction with metallic sodium at 850°C because the potassium is removed by distillation (see Le Chatelier's principle):
- KCl(l) + Na(l) ⇌ NaCl(l) + K(g)
This method is the main method for producing metallic potassium. Electrolysis (used for sodium) fails because of the high solubility of potassium in molten KCl.
As with other compounds containing potassium, KCl in powdered form gives a lilac flame test result.
Physical properties
Potassium chloride has a crystalline structure like many other salts. Its structure is face-centered cubic. Its lattice constant is roughly 630 picometers. Some other properties are
- Transmission range: 210 nm to 20 µm
- Transmittivity = 92% at 450 nm and rises linearly to 94% at 16 µm
- Refractive index = 1.456 at 10 µm
- Reflection Loss = 6.8% at 10 µm (two surfaces)
- dN/dT (expansion coefficient)= −33.2×10/°C
- dL/dT (refractive index gradient)= 40×10/°C
- Thermal conductivity = 0.036 W/(cm·K)
- Damage threshold (Newman & Novak): 4 GW/cm or 2 J/cm (0.5 or 1 ns pulse rate); 4.2 J/cm (1.7 ns pulse rate Kovalev & Faizullov)
Production


Potassium chloride occurs naturally as sylvite, and it can be extracted from sylvinite. It is also extracted from salt water and can be manufactured by crystallization from solution, flotation or electrostatic separation from suitable minerals. It is a by-product of the making of nitric acid from potassium nitrate and hydrochloric acid.
Uses
The majority of the potassium chloride produced is used for making fertilizer, since the growth of many plants is limited by their potassium intake. As a chemical feedstock, it is used for the manufacture of potassium hydroxide and potassium metal. It is also used in medicine, lethal injections, scientific applications, food processing, and as a sodium-free substitute for table salt (sodium chloride).
It is sometimes used in water as a completion fluid in petroleum and natural gas operations, as well as being an alternative to sodium chloride in household water softener units. KCl is useful as a beta radiation source for calibration of radiation monitoring equipment, because natural potassium contains 0.0118% of the isotope K. One kilogram of KCl yields 16350 becquerels of radiation consisting of 89.28% beta and 10.72% gamma with 1.46083 MeV. Potassium chloride is used in some Deicing products that are designed to be safer for pets and plants, though these are inferior in melting quality to calcium chloride (lowest usable temperature 0 °F (−18 °C) v. −25 °F (−32 °C)). It is also used in various brands of bottled water, as well as in bulk quantities for fossil fuel drilling purposes.
Potassium chloride was once used as a fire extinguishing agent, used in portable and wheeled fire extinguishers. Known as Super-K dry chemical, it was more effective than sodium bicarbonate-based dry chemicals and was compatible with protein foam. This agent fell out of favor with the introduction of potassium bicarbonate (Purple-K) dry chemical in the late 1960s, which was much less corrosive and more effective. It is rated for B and C fires.
Along with sodium chloride and lithium chloride, potassium chloride is used as a flux for the gas welding of aluminium.
Potassium chloride is also an optical crystal with a wide transmission range from 210 nm to 20 µm. While cheap, KCl crystal is hygroscopic. This limits its application to protected environments or short term uses such as prototyping. Exposed to free air, KCl optics will "rot". Whereas KCl components were formerly used for infrared optics, it has been entirely replaced by much tougher crystals like ZnSe.
Potassium chloride has also been used to create heat packs which employ exothermic chemical reactions, but these are no longer being created due to cheaper and more efficient methods, such as the oxidation of metals ('Hot Hands', one time use products) or the crystallization of sodium acetate (multiple use products).
Potassium chloride is used as a scotophor with designation P10 in dark-trace CRTs, e.g. in the Skiatron.
Biological and medical properties
Potassium is vital in the human body, and oral potassium chloride is the common means to replenish it, although it can also be diluted and given intravenously. It can be used as a salt substitute for food, but due to its weak, bitter, unsalty flavour, it is usually mixed with ordinary salt (sodium chloride) for this purpose to improve the taste. The addition of 1 ppm of thaumatin considerably reduces this bitterness. Medically, it is used in the treatment of hypokalemia and associated conditions, for digitalis poisoning, and as an electrolyte replenisher. Brand names include K-Dur, Klor-Con, Micro-K, Slow-K and Kaon Cl. Side effects can include gastrointestinal discomfort including nausea and vomiting, diarrhea and bleeding of the digestive tract. Overdoses cause hyperkalemia, which can lead to paresthesia, cardiac conduction blocks, fibrillation, arrhythmias, and sclerosis. Prescription potassium citrate (the potassium naturally found in fruits and vegetables) can be prescribed as an alternative to potassium chloride. Slow-K is a 1950s development where the medicine is formulated to enter the bloodstream at delayed intervals. It was first only prescribed to British military forces to balance their diets while serving in Korea.
Some cardiac surgery procedures cannot be carried out on the beating heart. For these procedures, the surgical team will bypass the heart with a heart-lung machine and inject potassium chloride into the heart muscle to stop the heartbeat.
The lethal effects of potassium chloride overdoses have led to its use in lethal injection, as the third of a three-drug combination. Additionally, KCl is used (albeit rarely) in fetal intracardiac injections in second- and third-trimester induced abortions. Jack Kevorkian's thanatron machine injected a lethal dose of potassium chloride into the patient, which caused the heart to stop functioning, after a sodium thiopental-induced coma was achieved. A similar device, the German 'Perfusor', also uses potassium chloride as a suicide aid.
Precautions
Orally, potassium chloride is toxic in excess; the LD50 is around 2.5 g/kg (meaning that a lethal dose for 50% of people weighing 75 kg (165 lb) is about 190 g (6.7 ounces)). Intravenously, this is reduced to just over 30 mg/kg, but of more concern are its severe effects on the cardiac muscles: high doses can cause cardiac arrest and rapid death, thus the aforementioned use as the third and final drug delivered in the lethal injection process.
References
- "Potassium chloride (PIM 430)". International Programme on Chemical Safety. 3.3.1 Properties of the substance. Retrieved 2011-01-17.
- ^ Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 061894690X.
- Material Safety Data Sheet – Potassium Chloride. Sigma–Aldrich. July 2001.
- Burgess, J. (1978). Metal Ions in Solution. New York: Ellis Horwood. ISBN 0-85312-027-7.
- U.S. patent 3,874,504
- Lorient, Denis; Linden, G. (1999). New ingredients in food processing: biochemistry and agriculture. Boca Raton: CRC Press. p. 357. ISBN 1-85573-443-5.
... in dietary food containing potassium chloride, thaumatin added in the ratio of 1 ppm considerably reduces the sensation of bitterness. ...
{{cite book}}:|access-date=requires|url=(help); Check date values in:|accessdate=(help)CS1 maint: multiple names: authors list (link) - Hypokalemia: Treatment & Medication. Emedicine.medscape.com. Retrieved on 2012-02-16.
- Hyperkalemia. Emedicine.medscape.com. Retrieved on 2012-02-16.
- He, F. J.; Markandu, ND; Coltart, R; Barron, J; MacGregor, GA (2005). "Effect of Short-Term Supplementation of Potassium Chloride and Potassium Citrate on Blood Pressure in Hypertensives". Hypertension. 45 (4): 571–4. doi:10.1161/01.HYP.0000158264.36590.19. PMID 15723964.
- Stubblefield PG; Carr-Ellis, S; Borgatta, L (2004). "Methods for induced abortion". Obstetrics and Gynecology. 104 (1): 174–85. doi:10.1097/01.AOG.0000130842.21897.53. PMID 15229018.
{{cite journal}}: Unknown parameter|month=ignored (help) - Types of Abortion Procedures. Americanpregnancy.org (2011-09-20). Retrieved on 2012-02-16.
- Boyes, Roger (2008-03-29). "Death for hire – suicide machine lets you push final button". The Times. Retrieved 2008-04-25.
- "Perfusor compact" (PDF).
- Fatal poisoning by potassium in human and rabbit Forensic Sci. vol. 9(1) p. 33-6, 1977.
- Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton, Florida: CRC Press. ISBN 0-8493-0486-5.
- Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. ISBN 978-0-08-022057-4.
| Potassium compounds | |
|---|---|
| H, (pseudo)halogens | |
| chalcogens | |
| pnictogens | |
| B, C group | |
| transition metals | |
| organic | |