This is an old revision of this page, as edited by Panamitsu (talk | contribs) at 01:29, 8 August 2023 (cleanup). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 01:29, 8 August 2023 by Panamitsu (talk | contribs) (cleanup)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) New Zealand physicist (1871–1937) "Lord Rutherford" redirects here. Not to be confused with Lord Rutherfurd or Andrew Rutherford, 1st Earl of Teviot.
Ernest Rutherford, 1st Baron Rutherford of Nelson, OM, PRS, HonFRSE (30 August 1871 – 19 October 1937) was a New Zealand physicist who was a pioneering researcher in both atomic and nuclear physics. Rutherford has been described as "the father of nuclear physics", and the greatest experimentalist "since Michael Faraday". In 1908, he was awarded the Nobel Prize in Chemistry "for his investigations into the disintegration of the elements, and the chemistry of radioactive substances." He was the first Oceanian Nobel laureate, and the first to perform the awarded work in Canada.
Rutherford's discoveries include the concept of radioactive half-life, the radioactive element radon, and the differentiation and naming of alpha and beta radiation. Together with Thomas Royds, Rutherford is credited with proving that alpha radiation is helium nuclei. In 1911, he theorized that atoms have their charge concentrated in a very small nucleus, and thereby pioneered the Rutherford model of the atom, through his discovery and interpretation of Rutherford scattering by the gold foil experiment of Hans Geiger and Ernest Marsden. He performed the first artificially induced nuclear reaction in 1917 in experiments where nitrogen nuclei were bombarded with alpha particles. As a result, he discovered the emission of a subatomic particle which he initially called the "hydrogen atom," but later (more accurately) named the proton. Later, Rutherford capped off his exploration of the nucleus with the discovery of the neutron. He is also credited with developing the atomic numbering system alongside Henry Moseley. His other achievements include advances in radio communications and ultrasound technology.
Rutherford became Director of the Cavendish Laboratory at the University of Cambridge in 1919. Under his leadership, the neutron was discovered by James Chadwick in 1932. In the same year, the first experiment to split the nucleus in a fully controlled manner was performed by students working under his direction, John Cockcroft and Ernest Walton.
In honor of his scientific advancements, Rutherford was recognized as a Baron in the peerages of both New Zealand and Britain. After his death in 1937, he was buried in Westminster Abbey near Charles Darwin and Isaac Newton. The chemical element rutherfordium (104Rf) was named after him in 1997.
Early life and education
Ernest Rutherford was born 30 August 1871 in Brightwater, a town near Nelson, New Zealand. He was the fourth of twelve children to James Rutherford, an immigrant farmer from Perth, Scotland, and his wife Martha Thompson, a schoolteacher from Hornchurch, England. Rutherford's birth certificate reads 'Earnest', possibly due to a clerical error. He studied at Havelock School, then in 1887, on his second attempt, won a scholarship to study at Nelson College. On his first examination attempt, he received 75 out of 130 marks for geography, 76 out of 130 for history, 101 out of 140 for english, and 200 out of 200 for arithmetic, totalling 452 out of 600 marks. With these marks, he had the highest of anyone from Nelson. When he was awarded the scholarship, he received 580 out of 600 possible marks. After being awarded the scholarship, Havelock School presented him with a five volume book, titled "The Peoples of the World". He was also offered a cadetship in government service, but he declined as he still had 15 months of college remaining. After his second attempt, in 1889, he won another scholarship to study at Canterbury College, University of New Zealand, where he participated in the debating society, was head boy and played rugby. He studied there between 1890 and 1894. At Canterbury, he was awarded a complex BA in Latin, English, and Maths in 1892, a MA in Mathematics and Physical Science in 1893, and a BSc in Chemistry and Geology in 1894.
Thereafter, he invented a new form of radio receiver, and in 1895 Rutherford was awarded an 1851 Research Fellowship from the Royal Commission for the Exhibition of 1851, to travel to England for postgraduate study at the Cavendish Laboratory, University of Cambridge. In 1897, he was awarded a BA Research Degree and the Coutts-Trotter Studentship from Trinity College, Cambridge.
Scientific career

When Rutherford began his studies at Cambridge, he was among the first 'aliens' (those without a Cambridge degree) allowed to do research at the university, and was additionally honoured to study under the mentorship of J. J. Thomson.
With Thomson's encouragement, he detected radio waves at 0.5 miles (800 m), and briefly held the world record for the distance over which electromagnetic waves could be detected, though when he presented his results at the British Association meeting in 1896, he discovered he had been outdone by Guglielmo Marconi, whose radio waves had sent a message across nearly 10 miles (16 km).
Work with radioactivity
Again under Thomson's leadership, Rutherford had worked on the conductive effects of X-rays on gases, which led to the discovery of the electron, first presented by Thomson in 1897. Hearing of Henri Becquerel's experience with uranium, Rutherford started to explore its radioactivity, discovering two types that differed from X-rays in their penetrating power. Continuing his research in Canada, he coined the terms alpha ray and beta ray in 1899 to describe the two distinct types of radiation.
In 1898, Rutherford was accepted to the chair of Macdonald Professor of physics position at McGill University in Montreal, Canada, on Thomson's recommendation. In 1901, he earned a DSc from the University of New Zealand.
From 1900 to 1903, he was joined at McGill by the young chemist Frederick Soddy (Nobel Prize in Chemistry, 1921) for whom he set the problem of identifying the noble gas emitted by the radioactive element thorium, a substance which was itself radioactive and would coat other substances. Once he had eliminated all the normal chemical reactions, Soddy suggested that it must be one of the inert gases, which they named thoron. This substance was later found to be Rn, an isotope of radon. They also found another substance they called Thorium X, later identified as Rn, and kept on finding traces of helium. They also worked with samples of "Uranium X" (protactinium) from William Crookes and radium from Marie Curie. Rutherford further investigated thoron in conjunction with R.B. Owens and found that a sample of radioactive material of any size invariably took the same amount of time for half the sample to decay (in this case, 111⁄2 minutes), a phenomenon for which he coined the term "half-life."
Rutherford and Soddy published their "Law of Radioactive Change" to account for all their experiments. Until then, atoms were assumed to be the indestructible basis of all matter and although Curie had suggested that radioactivity was an atomic phenomenon, the idea of the atoms of radioactive substances breaking up was a radically new idea. Rutherford and Soddy demonstrated that radioactivity involved the spontaneous disintegration of atoms into other, as yet, unidentified matter.
In 1903, Rutherford considered a type of radiation discovered (but not named) by French chemist Paul Villard in 1900, as an emission from radium, and realised that this observation must represent something different from his own alpha and beta rays, due to its very much greater penetrating power. Rutherford therefore gave this third type of radiation the name of gamma ray. All three of Rutherford's terms are in standard use today – other types of radioactive decay have since been discovered, but Rutherford's three types are among the most common.
In 1904, Rutherford suggested that radioactivity provides a source of energy sufficient to explain the existence of the Sun for the many millions of years required for the slow biological evolution on Earth proposed by biologists such as Charles Darwin. The physicist Lord Kelvin had argued earlier for a much younger Earth based on the insufficiency of known energy sources, but Rutherford pointed out at a lecture attended by Kelvin that radioactivity could solve this problem. Later that year, he was elected as a member to the American Philosophical Society, and in 1907, he returned to Britain to take the chair of physics at the Victoria University of Manchester.
In Manchester, Rutherford continued his work with alpha radiation. In conjunction with Hans Geiger, he developed zinc sulfide scintillation screens and ionisation chambers to count alphas. By dividing the total charge they produced by the number counted, Rutherford decided that the charge on the alpha was two. In late 1907, Ernest Rutherford and Thomas Royds allowed alphas to penetrate a very thin window into an evacuated tube. As they sparked the tube into discharge, the spectrum obtained from it changed, as the alphas accumulated in the tube. Eventually, the clear spectrum of helium gas appeared, proving that alphas were at least ionised helium atoms, and probably helium nuclei. The Nobel Prize in Chemistry 1908 was awarded to Ernest Rutherford "for his investigations into the disintegration of the elements, and the chemistry of radioactive substances".
Model of the atom
See also: Rutherford–Bohr model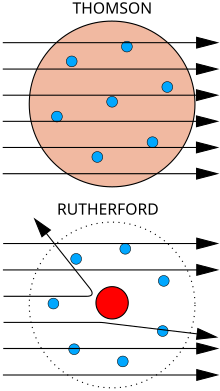
Bottom: Observed results: a small portion of the particles were deflected, indicating a small, concentrated charge. Diagram is not to scale; in reality the nucleus is vastly smaller than the electron shell.
Rutherford continued to make ground breaking discoveries long after receiving the Nobel prize in 1908. Along with Hans Geiger and Ernest Marsden in 1909, he carried out the Geiger–Marsden experiment, which demonstrated the nuclear nature of atoms by deflecting alpha particles passing through a thin gold foil. Rutherford was inspired to ask Geiger and Marsden in this experiment to look for alpha particles with very high deflection angles, of a type not expected from any theory of matter at that time. Such deflections, though rare, were found, and proved to be a smooth but high-order function of the deflection angle. It was Rutherford's interpretation of this data that led him to formulate the Rutherford model of the atom in 1911 – that a very small charged nucleus, containing much of the atom's mass, was orbited by low-mass electrons.
In 1912, Rutherford was joined by Niels Bohr (who postulated that electrons moved in specific orbits). Bohr adapted Rutherford's nuclear structure to be consistent with Max Planck's quantum theory, and the resulting Rutherford–Bohr model is considered valid to this day.
Piezoelectricity
During World War I, Rutherford worked on a top secret project to solve the practical problems of submarine detection. Both Rutherford and Paul Langevin suggested the use of piezoelectricity, and Rutherford successfully developed a device which measured its output. The use of piezoelectricity then became essential to the development of ultrasound as it is known today. The claim that Rutherford developed sonar, however, is a misconception, as subaquatic detection technologies utilize Langevin's transducer.
Discovery of the proton
Together with H.G. Moseley, Rutherford developed the atomic numbering system in 1913. Rutherford and Moseley's experiments used cathode rays to bombard various elements with a stream of electrons and observed that each element responded in a consistent and distinct manner. Their research was the first to assert that each element could be defined by the properties of its inner structures – an observation which later led to the discovery of the atomic nucleus. This research led Rutherford to theorize that the hydrogen atom (at the time the least massive entity known to bear a positive charge) was a sort of "positive electron" – a component of every atomic element.
It was not until 1919 that Rutherford expanded upon his research of the "positive electron" with a series of experiments beginning shortly before the end of his time at Manchester. He found that nitrogen and other light elements ejected a proton, which he called a "hydrogen atom", when hit with α (alpha) particles. In particular, he showed that particles ejected by alpha particles colliding with hydrogen have unit charge and 1/4 the momentum of alpha particles.
Rutherford returned to the Cavendish in 1919, succeeding J. J. Thomson as the Cavendish professor and Director, up until his death in 1937. During his tenure, Nobel Prizes were awarded to James Chadwick for discovering the neutron (in 1932), John Cockcroft and Ernest Walton for an experiment which was to be known as splitting the atom using a particle accelerator, and Edward Appleton for demonstrating the existence of the ionosphere.
Development of proton and neutron theory
In 1919–1920, Rutherford continued his research on the "hydrogen atom" to confirm that alpha particles breakdown nitrogen nuclei and affirm the nature of the products. This result showed Rutherford that hydrogen nuclei were a part of nitrogen nuclei (and by inference, probably other nuclei as well). Such a construction had been suspected for many years on the basis of atomic weights which were whole numbers of that of hydrogen; see Prout's hypothesis. Hydrogen was known to be the lightest element, and its nuclei presumably the lightest nuclei. Now, because of all these considerations, Rutherford decided that a hydrogen nucleus was possibly a fundamental building block of all nuclei, and also possibly a new fundamental particle as well, since nothing was known from the nucleus that was lighter. Thus, confirming and extending the work of Wilhelm Wien who in 1898 discovered the proton in streams of ionized gas, Rutherford postulated the hydrogen nucleus to be a new particle in 1920, which he dubbed the proton.
In 1921, while working with Niels Bohr, Rutherford theorized about the existence of neutrons, (which he had christened in his 1920 Bakerian Lecture), which could somehow compensate for the repelling effect of the positive charges of protons by causing an attractive nuclear force and thus keep the nuclei from flying apart from the repulsion between protons. The only alternative to neutrons was the existence of "nuclear electrons" which would counteract some of the proton charges in the nucleus, since by then it was known that nuclei had about twice the mass that could be accounted for if they were simply assembled from hydrogen nuclei (protons). But how these nuclear electrons could be trapped in the nucleus, was a mystery. Rutherford is widely quoted as saying, regarding the results of these experiments: "It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you."
Rutherford's theory of neutrons was proved in 1932 by his associate James Chadwick, who recognized neutrons immediately when they were produced by other scientists and later himself, in bombarding beryllium with alpha particles. In 1935, Chadwick was awarded the Nobel Prize in Physics for this discovery.
Re-evaluation of nuclear transmutation credit
A long-standing myth existed, at least as early as 1948, running at least to 2017, that Rutherford was the first scientist to observe and report an artificial transmutation of a stable element into another element: nitrogen into oxygen. It was thought by many people to be one of Rutherford's greatest accomplishments. The New Zealand government even issued a commemorative stamp in the belief that the nitrogen-to-oxygen discovery belonged to Rutherford. Beginning in 2017, many scientific institutions corrected their versions of this history to indicate that the discovery credit for the reaction belongs to Patrick Blackett. Rutherford did detect the ejected proton in 1919 and interpreted it as evidence for disintegration of the nitrogen nucleus (to lighter nuclei). In 1925, Blackett showed that the actual product is oxygen and identified the true reaction as N + α → O + p. Rutherford therefore recognized "that the nucleus may increase rather than diminish in mass as the result of collisions in which the proton is expelled".
Later years and honours
Rutherford received significant recognition in his home country of New Zealand, and was first awarded the Hector Memorial Medal in 1916. In 1925, Rutherford pushed calls to the New Zealand Government to support education and research, which led to the formation of the Department of Scientific and Industrial Research (DSIR) in the following year. Between 1925 and 1930, he served as President of the Royal Society, and later as president of the Academic Assistance Council which helped almost 1,000 university refugees from Germany. He was appointed to the Order of Merit in the 1925 New Year Honours and raised to the peerage as Baron Rutherford of Nelson, New Zealand and of Cambridge in the County of Cambridge in 1931, a title that became extinct upon his unexpected death in 1937. In 1933, Rutherford was one of the two inaugural recipients of the T. K. Sidey Medal, set up by the Royal Society of New Zealand as an award for outstanding scientific research.
Additionally, Rutherford received a number of awards from the British Crown. He was knighted in 1914, awarded an Order of Merit in 1925, and made a Baron in 1931, decorating his coat of arms with a kiwi and a Māori warrior.
Personal life and death
In 1900, Rutherford married Mary Georgina Newton (1876–1954), to whom he had become engaged before leaving New Zealand, at St Paul's Anglican Church, Papanui in Christchurch. They had one daughter, Eileen Mary (1901–1930), who married the physicist Ralph Fowler. Beyond science, Rutherford's hobbies included golf and motoring.
For some time before his death, Rutherford had a small hernia, which he had neglected to have fixed, and it became strangulated, rendering him violently ill. Despite an emergency operation in London, he died four days afterwards at age 66 of what physicians termed "intestinal paralysis", at Cambridge on 19 October 1937. After cremation at Golders Green Crematorium, he was given the high honour of burial in Westminster Abbey, near Isaac Newton and other illustrious British scientists such as Charles Darwin.
Legacy

Rutherford is considered to be among the greatest scientists in history. At the opening session of the 1938 Indian Science Congress, which Rutherford had been expected to preside over before his death, astrophysicist James Jeans spoke in his place and deemed him "one of the greatest scientists of all time", saying:
In his flair for the right line of approach to a problem, as well as in the simple directness of his methods of attack, often reminds us of Faraday, but he had two great advantages which Faraday did not possess, first, exuberant bodily health and energy, and second, the opportunity and capacity to direct a band of enthusiastic co-workers. Great though Faraday's output of work was, it seems to me that to match Rutherford's work in quantity as well as in quality, we must go back to Newton. In some respects he was more fortunate than Newton. Rutherford was ever the happy warrior – happy in his work, happy in its outcome, and happy in its human contacts.
Nuclear physics
Rutherford is known as "the father of nuclear physics" because his research, and work done under him as laboratory director, established the nuclear structure of the atom and the essential nature of radioactive decay as a nuclear process. Patrick Blackett, a research fellow working under Rutherford, using natural alpha particles, demonstrated induced nuclear transmutation. Rutherford's team later, using protons from an accelerator, demonstrated artificially-induced nuclear reactions and transmutation.
Rutherford died too early to see Leó Szilárd's idea of controlled nuclear chain reactions come into being. However, a speech of Rutherford's about his artificially-induced transmutation in lithium, printed on 12 September 1933 London paper The Times, was reported by Szilárd to have been his inspiration for thinking of the possibility of a controlled energy-producing nuclear chain reaction.
Rutherford's speech touched on the 1932 work of his students John Cockcroft and Ernest Walton in "splitting" lithium into alpha particles by bombardment with protons from a particle accelerator they had constructed. Rutherford realized that the energy released from the split lithium atoms was enormous, but he also realized that the energy needed for the accelerator, and its essential inefficiency in splitting atoms in this fashion, made the project an impossibility as a practical source of energy (accelerator-induced fission of light elements remains too inefficient to be used in this way, even today). Rutherford's speech in part, read:
We might in these processes obtain very much more energy than the proton supplied, but on the average we could not expect to obtain energy in this way. It was a very poor and inefficient way of producing energy, and anyone who looked for a source of power in the transformation of the atoms was talking moonshine. But the subject was scientifically interesting because it gave insight into the atoms.
The element rutherfordium, Rf, Z=104, was named in honor of Rutherford in 1997.
Publications
- Radio-activity (1904), 2nd ed. (1905), ISBN 978-1-60355-058-1
- Radioactive Transformations (1906), ISBN 978-1-60355-054-3
- Radioaktive Substanzen und ihre Strahlungen. Cambridge: University press. 1933.
- Radioaktive Substanzen und ihre Strahlungen (in German). Leipzig: Akademische Verlaggesellschaft. 1913.
- Radioactive Substances and their Radiations (1913)
- The Electrical Structure of Matter (1926)
- The Artificial Transmutation of the Elements (1933)
- The Newer Alchemy (1937)
Articles
- "Disintegration of the Radioactive Elements" Harper's Monthly Magazine, January 1904, pages 279 to 284.
See also
- Bateman equation
- Hydrophone
- Magnetic detector
- Neutron generator
- Royal Society of New Zealand
- Rutherford (unit)
- Rutherfordine
- The Rutherford Journal
- List of presidents of the Royal Society
Footnotes
- See the article on Kelvin for details of his arguments.
References
- ^ Ernest Rutherford and Frederick Soddy American Physical Society 2017
- Grodzins, Lee (February 1994). "Obituaries: Zhang Wen-Yu". Physics Today. 47 (2): 116. doi:10.1063/1.2808417. Retrieved 28 January 2023.
Zhang studied under Ernest Rutherford in the mid-1930s, receiving his degree from Cambridge University in 1938.
- Zhang Wenyu (张文裕) (28 March 2018). 高能实验物理学家张文裕:回忆导师卢瑟福生命中的最后两年. thepaper.com (in Chinese). Retrieved 12 August 2021.
- Eve, A. S.; Chadwick, J. (1938). "Lord Rutherford 1871–1937". Obituary Notices of Fellows of the Royal Society. 2 (6): 394. doi:10.1098/rsbm.1938.0025.
- ^ "Ernest Rutherford". Environmental Health and Safety Office of Research Regulatory Support. Michigan State University. Retrieved 23 June 2023.
- ^ Badash, Lawrence. "Ernest Rutherford | Accomplishments, Atomic Theory, & Facts | Britannica". Encyclopedia Britannica. Retrieved 23 June 2023.
- Campbell, John. "Rutherford – A Brief Biography". Rutherford.org.nz. Retrieved 4 March 2013.
- Rutherford, E.; Royds, T. (1908). "Spectrum of the radium emanation". Philosophical Magazine. Series 6. 16 (92): 313. doi:10.1080/14786440808636511.
- Longair, M. S. (2003). Theoretical concepts in physics: an alternative view of theoretical reasoning in physics. Cambridge University Press. pp. 377–378. ISBN 978-0-521-52878-8.
- Rutherford, E. (1919). "Collision of α particles with light atoms. IV. An anomalous effect in nitrogen". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. Series 6. 37 (222): 581–587. doi:10.1080/14786440608635919.
- Rutherford, E. (1920). "Bakerian Lecture. Nuclear Constitution of Atoms". Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences. 97 (686): 374–400. Bibcode:1920RSPSA..97..374R. doi:10.1098/rspa.1920.0040.
- ^ A.H. McLintock (18 September 2007). "Rutherford, Sir Ernest (Baron Rutherford of Nelson, O.M., F.R.S.)". An Encyclopaedia of New Zealand (1966 ed.). Te Ara – The Encyclopedia of New Zealand. ISBN 978-0-478-18451-8. Retrieved 2 April 2008.
- J.L. Heilbron. Ernest Rutherford And the Explosion of Atoms. Oxford University Press. p. 12. ISBN 0-19-512378-6.
- Campbell, John. "Rutherford, Ernest 1871–1937". Dictionary of New Zealand Biography. Ministry for Culture and Heritage. Retrieved 4 April 2011.
- "Papers Past | Newspapers | Marlborough Express | 7 October 1886 | LOCAL AND GENERAL NEWS". paperspast.natlib.govt.nz. Retrieved 8 August 2023.
- ^ Campbell, John (30 October 2012). "Rutherford, Ernest". An Encyclopaedia of New Zealand. Te Ara – The Encyclopaedia of New Zealand. Retrieved 1 October 2013.
- "RESULTS OF NELSON SCHOLARSHIPS EXAMINATIONS FOR DECEMBER 1885". paperspast.natlib.govt.nz. Retrieved 8 August 2023.
- "THE Marlborough Express. PUBLISHED EVERY EVENING. MONDAY, DECEMBER 28, 1885. LOCAL AND GENERAL NEWS". paperspast.natlib.govt.nz. Retrieved 8 August 2023.
- "THE Marlborough Express. Published Every Evening WEDNESDAY, JANUARY 5, 1887. LOCAL AND GENERAL NEWS". paperspast.natlib.govt.nz. Retrieved 8 August 2023.
- "Papers Past | Newspapers | Marlborough Express | 25 January 1887 | LOCAL AND GENERAL NEWS". paperspast.natlib.govt.nz. Retrieved 8 August 2023.
- "Papers Past | Newspapers | Marlborough Express | 4 October 1887 | Marlborough Express. Published Every Evening..." paperspast.natlib.govt.nz. Retrieved 8 August 2023.
- ^ "Ernest Rutherford Biographical". The Nobel Prize. Nobel Prize Outreach AB. Archived from the original on 3 June 2023. Retrieved 13 June 2023.
- "Famous Canterbury graduate Ernest Rutherford turns 150". The University of Canterbury. 27 August 2021. Retrieved 3 July 2023.
- 1851 Royal Commission Archives
- ^ "Rutherford, Ernest (RTRT895E)". A Cambridge Alumni Database. University of Cambridge.
- Holmes, Jonathan (13 May 2022). "Marconi's first radio broadcast made 125 years ago". BBC News. Retrieved 16 June 2023.
- ^ "Know the scientist: Ernest Rutherford". The Hindu. 17 June 2021. Retrieved 23 June 2023.
- Buchwald, Jed Z.; Warwick, Andrew (30 January 2004). Histories of the electron: the birth of microphysics. Cambridge, Mass.: MIT Press. pp. 21–30. ISBN 0262524244. Retrieved 27 June 2023.
- ^ Trenn, Thaddeus J. (1976). "Rutherford on the Alpha-Beta-Gamma Classification of Radioactive Rays". Isis. 67 (1): 61–75. doi:10.1086/351545. JSTOR 231134. S2CID 145281124.
- McKown, Robin (1962). Giant of the Atom, Ernest Rutherford. Julian Messner Inc, New York. p. 57.
- Kragh, Helge (5 February 2012). "Rutherford, Radioactivity, and the Atomic Nucleus". arXiv:1202.0954 .
- Kragh, Helge (5 February 2012). "Rutherford, Radioactivity, and the Atomic Nucleus". arXiv:1202.0954 .
- England, P.; Molnar, P.; Righter, F. (January 2007). "John Perry's neglected critique of Kelvin's age for the Earth: A missed opportunity in geodynamics". GSA Today. 17 (1): 4–9. Bibcode:2007GSAT...17R...4E. doi:10.1130/GSAT01701A.1.
- "APS Member History". search.amphilsoc.org. Retrieved 28 June 2021.
- "Ernest Rutherford: Heritage Heroes at The University of Manchester". The University of Manchester. Retrieved 27 June 2023.
- Rutherford, E.; Geiger, H.; Bateman, H. (October 1910). "LXXVI. The probability variations in the distribution of α particles". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 20 (118): 698–707. doi:10.1080/14786441008636955.
- Rutherford, E.; Royds, T. (February 1909). "XXI. The nature of the α particle from radioactive substances". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 17 (98): 281–286. doi:10.1080/14786440208636599.
- "The Nobel Prize in Chemistry 1908". The Nobel Prize. The Nobel Foundation. Retrieved 2 April 2020.
- Pestka, Jessica (25 April 2017). "About Rutherford's Gold Foil Experiment". Sciencing. Retrieved 27 June 2023.
- Dragovich, Branko. Ernest Rutherford and the Discovery of the Atomic Nucleus (PDF). Belgrade: Institute of Physics. Retrieved 27 June 2023.
- Davidson, Michael W. (March 2014). "Pioneers in Optics: Johann Wilhelm Ritter and Ernest Rutherford" (PDF). Microscopy Today. 22 (2). Cambridge University Press: 48–51. doi:10.1017/S1551929514000029. Retrieved 27 June 2023.
- Rutherford, E. (1911). "The scattering of α and β particles by matter and the structure of the atom". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. Series 6. 21 (125): 669–688. doi:10.1080/14786440508637080.
- Katzir, Shaul (20 June 2012). "Who knew piezoelectricity? Rutherford and Langevin on submarine detection and the invention of sonar". Notes and Records of the Royal Society. 66 (2): 141–157. doi:10.1098/rsnr.2011.0049. Retrieved 2 July 2023.
- Duck, Francis (1 November 2022). "Paul Langevin, U-boats, and ultrasonics". Physics Today. 75 (11): 42–48. doi:10.1063/PT.3.5122. Retrieved 2 July 2023.
- Rutherford, Ernest (1914). "The structure of the atom" (PDF). Philosophical Magazine. 27: 488–498. Retrieved 13 June 2023.
- Whittaker, Edmund (1989). A History of the Theories of Aether and Electricity. Vol. 2. Courier Dover Publications. p. 87. ISBN 0-486-26126-3.
- Rutherford, Ernest (8 April 2009). "LII. Collision of α particles with light atoms II. Velocity of the hydrogen atom". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 6. 37 (222): 562–571. doi:10.1080/14786440608635917. Retrieved 13 June 2023.
- "The Cavendish Professorship of Physics". University of Cambridge. Archived from the original on 3 July 2013. Retrieved 30 November 2013.
- Wien, W. (1904). "Über positive Elektronen und die Existenz hoher Atomgewichte". Annalen der Physik. 318 (4): 669–677. Bibcode:1904AnP...318..669W. doi:10.1002/andp.18943180404.
- "Atop the Physics Wave: Rutherford back in Cambridge, 1919–1937". Rutherford's Nuclear World: The Story of the Discovery of the Nucleus. American Institute of Physics. Retrieved 26 June 2023.
- E. N. da C. Andrade, Rutherford and the Nature of the Atom (1964), cited in Ratcliffe, Susan, ed. (2016). "Ernest Rutherford 1871–1937, New Zealand physicist". Oxford Essential Quotations (4th ed.). ISBN 9780191826719.
- "James Chadwick - Facts". The Nobel Prize. Nobel Prize Outreach AB. Retrieved 16 June 2023.
- "Nobel Prize for Physics : Prof. P. M. S. Blackett, F.R.S". Nature. 162 (4126): 841. 1948. Bibcode:1948Natur.162R.841.. doi:10.1038/162841b0.
- "Exploring the atom". The Manhattan Project – Adventures Inside the Atom. U.S. Department of Energy – Office of History and Heritage Resources. Retrieved 19 June 2019.
- Dacey, James (1 September 2011). "What was Rutherford's greatest discovery?". Physics World. Retrieved 18 June 2019.
- Allibone, Thomas Edward (1964). "Rutherford Memorial Lecture, 1963 The industrial development of nuclear power". Proceedings of the Royal Society of London. Series A. Mathematical and Physical Sciences. 282 (1391): 447–463. Bibcode:1964RSPSA.282..447A. doi:10.1098/rspa.1964.0245. S2CID 97303563.
- Campbell, John (2009). "Inside Story: The genius of Rutherford revisited". CERN Courier. 49 (2): 46–48.
- "Exploring the atom". The Manhattan Project – an interactive history. U.S. Department of Energy – Office of History and Heritage Resources. Retrieved 18 June 2019.
- Rutherford, Sir Ernest (27 March 1925). "Studies of Atomic Nuclei". Science. 62 (1601). Physical Sciences Volume 9: The Royal Institution Library of Sciences: 73–76. Bibcode:1925Sci....62..209R. doi:10.1126/science.62.1601.209. PMID 17748045. Retrieved 29 June 2019.
{{cite journal}}: CS1 maint: location (link) - "Recipients". Royal Society Te Apārangi. Retrieved 16 February 2021.
- Brewerton, Emma (15 December 2014). "Ernest Rutherford". Ministry for Culture and Heritage.
- "No. 14089". The Edinburgh Gazette. 2 January 1925. p. 4.
- "No. 33683". The London Gazette. 23 January 1931. p. 533.
- "The Nobel Prize in Chemistry 1908". NobelPrize.org. Retrieved 16 April 2022.
- "Background of the Medal". Royal Society of New Zealand. Retrieved 7 August 2015.
- "Recipients". Royal Society of New Zealand. Retrieved 7 August 2015.
- "Ernest Rutherford - Biography". New Zealand History. Retrieved 23 June 2023.
- "No. 12647". The Edinburgh Gazette. 27 February 1914. p. 269.
- Intergen. "General". www.bdmhistoricalrecords.dia.govt.nz. Retrieved 8 February 2023.
- "Family history in from the cold". 18 March 2009.
- Summerfield, Fiona (9 November 2012). "Historic St Paul's Church in the Christchurch suburb of Papanui is being fully restored". Anglican Taonga.
- ^ The Complete Peerage, Volume XIII – Peerage Creations, 1901–1938. St Catherine's Press. 1949. p. 495.
- Heilbron, J. L. (12 June 2003). Ernest Rutherford: And the Explosion of Atoms. Oxford University Press. pp. 123–124. ISBN 978-0-19-512378-4.
- "Viceroy Opens The Congress – Sir James Jeans's Address". The Times. Calcutta. 3 January 1938.
- "Ernest Rutherford: father of nuclear science". New Zealand Media Resources. Archived from the original on 12 June 2021.
- Giunta, Carmen (2019). "Rutherford and Blackett artificial transmutation". web.lemoyne.edu. Retrieved 27 June 2023.
- "September 12, 1933 – Leó Szilárd conceives the idea of the nuclear chain reaction". Rincón educativo (in Spanish and English). Retrieved 27 June 2023.
- "The British association – breaking down the atom". The Times. 12 September 1933.
- Rhodes, Richard (1986). The Making of the Atomic Bomb. New York: Simon and Schuster. p. 27. ISBN 0-671-44133-7.
- Freemantle, Michael (2003). "ACS Article on Rutherfordium". Chemical & Engineering News. American Chemical Society. Retrieved 2 April 2008.
- "Review of Radio-activity by Ernest Rutherford". The Oxford Magazine. 23. The Proprietors: 347. 25 January 1905.
- Carmichael, R. D. (1916). "Book Review: Radioactive Substances and their Radiations" (PDF). Bulletin of the American Mathematical Society. 22 (4): 200. doi:10.1090/s0002-9904-1916-02762-5.
Further reading
- Badash, Lawrence (2008) . "Rutherford, Ernest". Oxford Dictionary of National Biography (online ed.). Oxford University Press. doi:10.1093/ref:odnb/35891. (Subscription or UK public library membership required.)
- Cragg, R. H. (1971). "Lord Ernest Rutherford of Nelson (1871–1937)". Royal Institute of Chemistry, Reviews. 4 (2): 129. doi:10.1039/RR9710400129.
- Campbell, John. (1999) Rutherford: Scientist Supreme, AAS Publications, Christchurch, ISBN 0-4730-5700-X
- Marsden, E. (1954). "The Rutherford Memorial Lecture, 1954. Rutherford-His Life and Work, 1871–1937". Proceedings of the Royal Society A. 226 (1166): 283–305. Bibcode:1954RSPSA.226..283M. doi:10.1098/rspa.1954.0254. S2CID 73381519.
- Reeves, Richard (2008). A Force of Nature: The Frontier Genius of Ernest Rutherford. New York: W. W. Norton. ISBN 0-393-33369-8
- Rhodes, Richard (1986). The Making of the Atomic Bomb. New York: Simon & Schuster. ISBN 0-671-44133-7
- Wilson, David (1983). Rutherford. Simple Genius, Hodder & Stoughton, ISBN 0-340-23805-4
External links
- Biography and web exhibit from the American Institute of Physics
- Ernest Rutherford on Nobelprize.org
 including the Nobel Lecture, 11 December 1908 The Chemical Nature of the Alpha Particles from Radioactive Substances
including the Nobel Lecture, 11 December 1908 The Chemical Nature of the Alpha Particles from Radioactive Substances - The Rutherford Museum
- Rutherford Scientist Supreme
- Newspaper clippings about Ernest Rutherford in the 20th Century Press Archives of the ZBW
| Academic offices | ||
|---|---|---|
| Preceded byArthur Schuster | Langworthy Professor at the University of Manchester 1907–1919 |
Succeeded byLawrence Bragg |
| 1908 Nobel Prize laureates | |
|---|---|
| Chemistry |
|
| Literature (1908) |
|
| Peace |
|
| Physics |
|
| Physiology or Medicine |
|
| Dalton Medallists | |
|---|---|
| Recipients |
|
 Media from Commons
Media from Commons Quotations from Wikiquote
Quotations from Wikiquote Texts from Wikisource
Texts from Wikisource
- Ernest Rutherford
- 1871 births
- 1937 deaths
- Experimental physicists
- New Zealand physicists
- New Zealand nuclear physicists
- Radio pioneers
- Nobel laureates in Chemistry
- Recipients of the Copley Medal
- Academic staff of McGill University
- Presidents of the Royal Society
- New Zealand Fellows of the Royal Society
- Foreign associates of the National Academy of Sciences
- Members of the Pontifical Academy of Sciences
- Honorary Members of the Russian Academy of Sciences (1917–1925)
- Honorary Members of the USSR Academy of Sciences
- Fellows of Trinity College, Cambridge
- University of Canterbury alumni
- Academics of the Victoria University of Manchester
- Barons in the Peerage of the United Kingdom
- Knights Bachelor
- People from Brightwater
- New Zealand people of English descent
- New Zealand people of Scottish descent
- British Nobel laureates
- English Nobel laureates
- New Zealand Nobel laureates
- Burials at Westminster Abbey
- Fellows of the Royal Society of New Zealand
- Persons of National Historic Significance (Canada)
- People educated at Nelson College
- Presidents of the Institute of Physics
- Honorary Fellows of the Royal Society of Edinburgh
- Corresponding Members of the Russian Academy of Sciences (1917–1925)
- Barons created by George V
- New Zealand recipients of a British peerage
- New Zealand emigrants to the United Kingdom
- 20th-century British physicists
- 19th-century British physicists
- 20th-century British scientists
- 19th-century New Zealand scientists
- New Zealand members of the Order of Merit
- Recipients of the Matteucci Medal
- Recipients of the Dalton Medal
- Members of the American Philosophical Society
- Discoverers of chemical elements
- Cavendish Professors of Physics

