This is the current revision of this page, as edited by FrescoBot (talk | contribs) at 15:47, 5 September 2023 (Bot: link syntax). The present address (URL) is a permanent link to this version.
Revision as of 15:47, 5 September 2023 by FrescoBot (talk | contribs) (Bot: link syntax)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)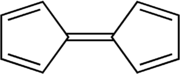
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name -2,2′,4,4′-tetraene | |
| Other names
Bicyclopentyliden-2,4,2′,4′-tetraene 1,1′-Bi Pentafulvalene Bicyclopentadienylidene Bicyclopentadienylidene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C10H8 |
| Molar mass | 128.174 g·mol |
| Density | 1.129 g/ml |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Fulvalene (bicyclopentadienylidene) is the member of the fulvalene family with the molecular formula C10H8. It is of theoretical interest as one of the simplest non-benzenoid conjugated hydrocarbons. Fulvalene is an unstable isomer of the more common benzenoid aromatic compounds naphthalene and azulene. Fulvalene consists of two 5-membered rings, each with two double bonds, joined by yet a fifth double bond. It has D2h symmetry.
History
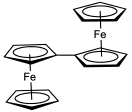
An earlier attempt at synthesis of fulvalene in 1951 by Pauson and Kealy resulted in the accidental discovery of ferrocene. Its synthesis was first reported in 1958 by E. A. Matzner, working under William von Eggers Doering. In this method, the cyclopentadienyl anion is coupled with iodine to the dihydrofulvalene. Double deprotonation of the dihydrofulvalene with n-butyllithium gives the dilithio derivative, which is oxidized by oxygen. Fulvalene was spectroscopically observed at −196 °C (77 K) from photolysis of diazocyclopentadiene, which induces dimerization of cyclopentadiene-derived carbenes. The compound was isolated in 1986 and was found to be nonaromatic. Above −50 °C (223 K) it dimerizes by a Diels–Alder reaction.
Derivatives
Perchlorofulvalene (C4Cl4C)2 is quite stable in contrast to fulvalene itself.
See also
- Fulvenes, (CH=CH)2C=CH2 and substituted derivatives
- Tetrathiafulvalene, C2H2S2C=CS2C2H2
References
- T. J. Kealy, P. L. Pauson (1951). "A New Type of Organo-Iron Compound". Nature. 168 (4285): 1039–1040. Bibcode:1951Natur.168.1039K. doi:10.1038/1681039b0. S2CID 4181383.
- Dissertation Abstracts Int'l 26-06 page 3270 6411876.
- Demore, William B.; Pritchard, H. O.; Davidson, Norman (1959). "Photochemical Experiments in Rigid Media at Low Temperatures. II. The Reactions of Methylene, Cyclopentadienylene and Diphenylmethylene". Journal of the American Chemical Society. 81 (22): 5874–5879. doi:10.1021/ja01531a008.
- Escher, André; Rutsch, Werner; Neuenschwander, Markus (1986). "Synthese von Pentafulvalen durch oxidative Kupplung von Cyclopentadienid mittels Kupfer(II)-chlorid". Helvetica Chimica Acta. 69 (7): 1644–1654. doi:10.1002/hlca.19860690719.
- Mark, V. (1966). "Perchlorofulvalene". Organic Syntheses. 46: 93. doi:10.15227/orgsyn.046.0093.