This is an old revision of this page, as edited by Citation bot 1 (talk | contribs) at 03:13, 27 September 2011 (Add: author-separator, author2, author3, display-authors, last4, first4, last5, first5, last6, first6, last7, first7, last8, first8, last9, first9. Tweak: author. You can use this bot yourself. Report bugs here.). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 03:13, 27 September 2011 by Citation bot 1 (talk | contribs) (Add: author-separator, author2, author3, display-authors, last4, first4, last5, first5, last6, first6, last7, first7, last8, first8, last9, first9. Tweak: author. You can use this bot yourself. Report bugs here.)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound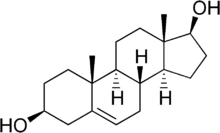 | |
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.553 |
| Chemical and physical data | |
| Formula | C19H30O2 |
| Molar mass | 290.44 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
5-Androstenediol (androst-5-ene-3beta,17beta-diol or 5-AED) is one of two androstenediols. Its potential use as a radiation countermeasure was introduced by the Armed Forces Radiobiology Research Institute (AFRRI) and subsequently studied by AFRRI and Hollis-Eden Pharmaceuticals under the tradename Neumune for the treatment of acute radiation syndrome.
The clinical trials on rhesus monkeys were successful. According to the Hollis-Eden report, only 12.5% of the 40 Neumune-treated animals died versus 32.5% in the placebo group.
Hollis-Eden had applied for a contract from the US Government under the BioShield Request for Proposals (RFP) for radiation countermeasures. After being encouraged for 2.5 years that Neumune was in the competitive range, on March 9, 2007, the RFP was canceled by HHS. According to HHS, "the product was no longer in the competitive range". No further explanation was given. As a result, Hollis-Eden has now withdrawn from the radiation countermeasure field.
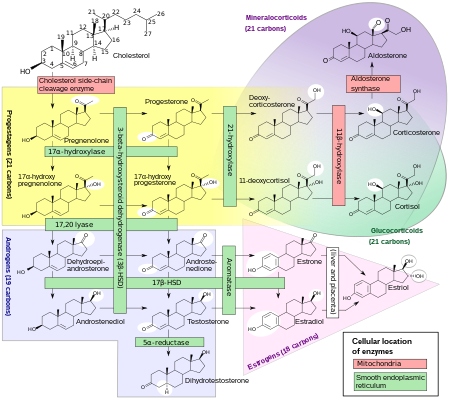
5-Androstenediol is a direct metabolite of the most abundant steroid produced by the human adrenal cortex, dehydroepiandrosterone (DHEA). 5-Androstenediol is less androgenic than 4-androstenediol, and stimulates the immune system. When administered to rats in vivo, 5-androstenediol has approximately 1/70 the androgenicity of DHEA, 1/185 the androgenicity of androstenedione, and 1/475 the androgenicity of testosterone. 5-AED's value as a radiation countermeasure is based mainly on its stimulation of production of white blood cells and platelets.
See also
References
- ^ Whitnall MH; Elliott TB; Harding RA; et al. (2000). "Androstenediol stimulates myelopoiesis and enhances resistance to infection in gamma-irradiated mice". Int. J. Immunopharmacol. 22 (1): 1–14. doi:10.1016/S0192-0561(99)00059-4. PMID 10684984.
{{cite journal}}: Unknown parameter|author-separator=ignored (help)CS1 maint: numeric names: authors list (link) - Hollis-Eden Pharmaceuticals Reports Publication of Results Demonstrating the Ability of NEUMUNE(R) to Increase Survival in a Primate Model of Lethal Radiation Injury, February 26, 2007.
- Government Nukes Hollis-Eden's Radiation Drug, by Val Brickates Kennedy and Angela Moore, March 8, 2007
- US cancels radiation contract with Hollis-Eden, March 9, 2007
- Coffey, DS (1988) "Androgen action and the sex accessory tissues". In E Knobil, J Neill (eds), The Physiology of Reproduction. Raven Press, New York, pp 1081-1119.
External links
- Regenerative Medicine - Official Hollis-Eden Pharmaceuticals website
- New Scientist article on AED as an anti-radiation sickness drug
| Cholesterol and steroid metabolic intermediates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mevalonate pathway |
| ||||||||||
| Non-mevalonate pathway | |||||||||||
| To Cholesterol | |||||||||||
| From Cholesterol to Steroid hormones |
| ||||||||||
| Nonhuman |
| ||||||||||