This is an old revision of this page, as edited by JWBE (talk | contribs) at 12:30, 29 October 2011 (→Precursor to other chemicals: ±Image). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 12:30, 29 October 2011 by JWBE (talk | contribs) (→Precursor to other chemicals: ±Image)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |||
| Names | |||
|---|---|---|---|
| IUPAC name 1,4-Dichlorobenzene | |||
| Other names
para-Dichlorobenzene p-Dichlorobenzene p-DCB PDB Paramoth Para crystals Paracide Moth Nuggets | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.092 | ||
| KEGG | |||
| RTECS number |
| ||
| UNII | |||
| CompTox Dashboard (EPA) | |||
SMILES
| |||
| Properties | |||
| Chemical formula | C6H4Cl2 | ||
| Molar mass | 147.00 g·mol | ||
| Density | 1.25 g/cm³, solid | ||
| Melting point | 53.5 °C (128.3 °F; 326.6 K) | ||
| Boiling point | 174 °C (345 °F; 447 K) | ||
| Solubility in water | 10.5 mg/100 mL (20 °C) | ||
| Hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | 66 °C | ||
| Related compounds | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
1,4-Dichlorobenzene (para-dichlorobenzene, p-DCB, PDB) is an organic compound with the formula C6H4Cl2. This colorless solid has a strong odor. It consists of two chlorine atoms substituted at opposing sites on a benzene ring. p-DCB is used a pesticide and a deodorant, most familiarly in mothballs in which it is a replacement for the more traditional naphthalene. p-DCB is also used as a precursor in the production of the polymer poly(p-phenylene sulfide).
Production
p-DCB is produced by chlorination of benzene using ferric chloride as a catalyst:
- C6H6 + 2 Cl2 → C6H4Cl2 + 2 HCl
The chief impurity is the 1,2 isomer. The compound can be purified by fractional crystallisation, taking advantage of its relatively high melting point of 53.5 °C; the isomeric dichlorobenzenes and chlorobenzene melt well below room temperature.
Uses
Disinfectant, deodorant, and pesticide
p-DCB is used to control moths, moulds, and mildew. It finds use as a disinfectant in waste containers and restrooms and is the characteristic smell associated with urinal cakes. Its usefulness in these arises from p-DCB's low solubility in water and its relatively high volatility: it sublimes readily near room temperature.
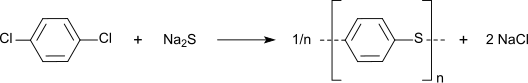
Precursor to other chemicals
The chlorides on p-DCB can be substituted with hydroxyl, amine, and sulfide groups. In a growing application, p-DCB is the precursor to the high performance polymer poly(p-phenylene sulfide):
Environmental effects
p-DCB is poorly soluble in water and is not easily broken down by soil organisms. Like many hydrocarbons, p-DCB is lipophilic and will accumulate in the fatty tissues.
Health effects
The US Department of Health and Human Services (DHHS) and the International Agency for Research on Cancer (IARC) have determined that p-DCB may reasonably be anticipated to be a carcinogen, although there is no direct evidence. Animals given very high levels in water developed liver and kidney tumors . The United States Environmental Protection Agency (EPA) has set a target maximum contaminant level of 75 micrograms of p-DCB per liter of drinking water (75 μg/L). p-DCB is also an EPA-registered pesticide. The US Occupational Safety and Health Administration (OSHA) has set a maximum level of 75 parts of p-DCB per million parts air in the workplace (75 ppm) for an 8-hour day, 40-hour workweek.
Little information is available on how children react to p-DCB exposure; however, consumption of p-DCB can cause vomiting in adults. A 2010 Japanese study found that the compound has anabolic-androgenic effect in rats and reduces sperm production although it does not bind to the androgen receptor, and neither does its major metabolite, 2,5-dichlorophenol.
Under California's Proposition 65, p-DCB is listed as "known to the State to cause cancer".
See also
References
- "National Pesticide Information Center - Mothballs Case Profile" (PDF). Retrieved 2009-08-10.
- ^ Manfred Rossberg, Wilhelm Lendle, Gerhard Pfleiderer, Adolf Tögel, Eberhard-Ludwig Dreher, Ernst Langer, Heinz Rassaerts, Peter Kleinschmidt, Heinz Strack, Richard Cook, Uwe Beck, Karl-August Lipper, Theodore R. Torkelson, Eckhard Löser, Klaus K. Beutel, “Chlorinated Hydrocarbons” in Ullmann’s Encyclopedia of Industrial Chemistry, 2006 John Wiley-VCH: Weinheim. doi:10.1002/14356007.a06 233.pub2
- Preamble to the IARC Monographs definition of "Group 2B: Possibly carcinogenic to humans", the International Agency for Research on Cancer classification of this chemical
- "Consumer Factsheet on: PARA-DICHLOROBENZENE (p-DCB)". November 28, 2006. Retrieved 2009-08-10.
- "Reregistration Eligibility Decision for Para-dichlorobenzene" (PDF). December 2008. Retrieved 2009-08-10.
- Woman Sickened by Chemical-Tainted Cup Noodle, Japan Today
- Takahashi, O; Ohashi, N; Nakae, D; Ogata, A (2011). "Parenteral paradichlorobenzene exposure reduces sperm production, alters sperm morphology and exhibits an androgenic effect in rats and mice". Food and chemical toxicology. 49 (1): 49–56. doi:10.1016/j.fct.2010.09.029. PMID 20932873.
- Proposition 65, Office of Environmental Health Hazard Assessment
External links
- International Chemical Safety Card 0037
- Mothball sniffing warning issued, BBC News, 27 July 2006