| |
| Names | |
|---|---|
| Preferred IUPAC name Cyclohexane-1,2-diamine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.010.707 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| UN number | 2735 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H14N2 |
| Molar mass | 114.192 g·mol |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H302, H312, H314, H317, H332, H335 |
| Precautionary statements | P260, P261, P264, P270, P271, P272, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P322, P330, P333+P313, P363, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
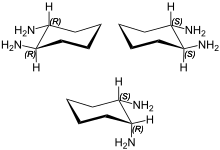
1,2-Diaminocyclohexane (DACH) is an organic compound with the formula (CH2)4(CHNH2)2. It is a mixture of three stereoisomers: cis-1,2-diaminocyclohexane and both enantiomers of trans-1,2-diaminocyclohexane. The mixture is a colorless, corrosive liquid, although older samples can appear yellow. It is often called DCH-99 and also DACH.
Manufacture
The product is available commercially, manufactured by the hydrogenation of o-phenylenediamine. The two trans enantiomers can be resolved by conversion to diastereomeric salts of various chiral acids.
Uses
The product is an epoxy curing agent for use in Coatings, Adhesives, Sealants and Elastomers - CASE. It is particularly useful in epoxy flooring. It may also be reacted with diethyl maleate utilizing the Michael reaction to produce a polyaspartic compound of CAS number 481040-92-0. It may also be used in lubricants. The product is also advertised as being useful as a chelating agent in a variety of applications including oil production. It also is used in downfield oil and gas wells where there is an acidic stream to prevent corrosion to the bore piles.
See also
- Hexamethylenediamine
- Isophorone diamine
- 1,3-BAC
- 2,3-Butanediamine, a vicinal diamine that also exists as three stereoisomers
References
- Kouklovsky, Cyrille; Langlois, Yves; Aguilar, Enrique; Fernández-García, Jesús M.; Sikervar, Vikas (2014). "(1S,2S)-1,2-Diaminocyclohexane". Encyclopedia of Reagents for Organic Synthesis. pp. 1–23. doi:10.1002/047084289x.rn00145.pub3. ISBN 978-0-470-84289-8.
- "Dytek DCH-99 by INVISTA - Paint & Coatings". www.ulprospector.com. Retrieved 2020-04-13.
- "A New Epoxy Curing Agent with Long Pot Life and Fast Cure". www.pcimag.com. Retrieved 2021-05-18.
- "Dytek DCH-99 | 1,2-Diaminocyclohexane". Dytek. Retrieved 2020-04-13.
- "US Patent for Non-aromatic based antioxidants for lubricants Patent (Patent # 9,273,266 issued March 1, 2016) - Justia Patents Search". patents.justia.com. Retrieved 2020-04-13.
- "Technical Data Sheet Dytek DCH 99" (PDF). Archived (PDF) from the original on 2020-08-19.
- Materials, Ascend. "FlexaTram-DAM". Ascend Performance Materials. Retrieved 2020-05-21.