| |
| Names | |
|---|---|
| Other names
1,2-Dimethylethylenediamine 2,3-Diaminobutane Butane-2,3-diamine | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) |
|
| ChemSpider |
|
| PubChem CID | |
| UNII |
|
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H12N2 |
| Molar mass | 88.154 g·mol |
| Appearance | colorless oil |
| Boiling point | 44-45 °C (25 mmHg, rac) 46-48 °C (25 mmHg, meso) 55.3-59.3 °C (60 mmHg, DL-threo) 56.1-60.5 °C (60 mmHg, meso) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
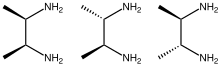
2,3-Butanediamine are organic compounds with the formula CH3CH(NH2)CH(NH2)CH3. Three stereoisomers exist, meso and a pair of enantiomers. These diamines form complexes with transition metals.
Synthesis
2,3-Butanediamines can be prepared by hydrolyzing 2-ethoxy-4,5-dihydro-4,5-dimethylimidazole with barium hydroxide. Alternative, it is produced by reduction of dimethylglyoxime with lithium aluminium hydride. The meso and the d,l diastereomers can be separated by fractional crystallization of the hydrochlorides. The enantiomers have been resolved using tartrate salts.
Reactions
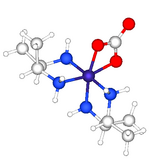
In coordination chemistry, 2,3-butanediamine (abbreviated bn) has illuminates aspects of the stereochemistry. The structure of confirms the presence of the rarely observed axial methyl groups on each of the diamine-cobalt rings.
Related compounds
- 1,2-Diaminopropane, chiral 1,2-diamine
- 1,2-Diaminocyclohexane, a 1,2-diamine that also exists as three stereoisomers
References
- Fred Basolo, R. Kent Murmann, and Yun Ti Chen. Dissociation Constants of Substituted Ethylenediamines. J. Am. Chem. Soc. 1953, 75, 6, 1478–1480. doi:10.1021/ja01102a507.
- Robert Ghirardelli and Howard J. Lucas. Stereochemistry of the Opening of the Imine Ring with Ethylamine. J. Am. Chem. Soc. 1957, 79, 3, 734–741. doi:10.1021/ja01560a064.
- Tsuchiya, Ryokichi; Uehara, Akira; Yoshikuni, Tadatsugu (1982). "Solid-phase thermal cis-trans isomerization of bis(diamine)chromium(III) complexes containing d,l-2,3-butanediamine, d,l-1,2-cyclohexanediamine, or d,l-2,4-pentanediamine". Inorganic Chemistry. 21 (2): 590–594. doi:10.1021/ic00132a025.
- Harold Kohn and Sang Hun Jung. New stereoselective method for the preparation of vicinal diamines from olefins and cyanamide. Journal of the American Chemical Society 1983 105 (12), 4106-4108. doi:10.1021/ja00350a068.
- Hilleary, Christopher J.; Them, Theodore F.; Tapscott, Robert E. (1980). "Stereochemical studies on diastereomers of tris(2,3-butanediamine)cobalt(III)". Inorganic Chemistry. 19: 102–107. doi:10.1021/ic50203a022.
- Dickey, F. H.; Fickett, Wildon; Lucas, H. J. (1952). "Stereoisomeric 2,3-Butanediamines, 3-Amino-2-butanols and 2,3-Dimethylethyleneimines; Stereochemistry of the Opening and Closing of the Imine Ring". Journal of the American Chemical Society. 74 (4): 944–951. doi:10.1021/ja01124a023.
- Duesler, E. N.; Fe Gargallo, M.; Tapscott, R. E. (1982). "Structure of lel lel lel tris[(±)-2,3-butanediamine]-cobalt(III) chloride". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 38 (4): 1300–1303. Bibcode:1982AcCrB..38.1300D. doi:10.1107/S0567740882005585.