| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1,2-Xylene | |||
| Systematic IUPAC name 1,2-Dimethylbenzene | |||
| Other names o-Xylene, o-Xylol | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 1815558 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.002.203 | ||
| EC Number |
| ||
| Gmelin Reference | 67796 | ||
| KEGG | |||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1307 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C8H10 | ||
| Molar mass | 106.168 g·mol | ||
| Appearance | Colorless liquid | ||
| Density | 0.88 g/ml | ||
| Melting point | −24 °C (−11 °F; 249 K) | ||
| Boiling point | 144.4 °C (291.9 °F; 417.5 K) | ||
| Solubility in water | 0.02% (20°C) | ||
| Solubility in ethanol | very soluble | ||
| Solubility in diethyl ether | very soluble | ||
| Vapor pressure | 7 mmHg (20°C) | ||
| Magnetic susceptibility (χ) | -77.78·10 cm/mol | ||
| Refractive index (nD) | 1.50545 | ||
| Viscosity | 1.1049 cP at 0 °C 0.8102 cP at 20 °C | ||
| Structure | |||
| Dipole moment | 0.64 D | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Mildly toxic | ||
| GHS labelling: | |||
| Pictograms |   
| ||
| Signal word | Danger | ||
| Hazard statements | H225, H226, H304, H305, H312, H315, H319, H332, H335, H412 | ||
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P273, P280, P301+P310, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P312, P321, P322, P331, P332+P313, P337+P313, P362, P363, P370+P378, P403+P233, P403+P235, P405, P501 | ||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | 32 °C (90 °F; 305 K) | ||
| Autoignition temperature |
463 °C (865 °F; 736 K) | ||
| Explosive limits | 0.9%-6.7% | ||
| Threshold limit value (TLV) | 100 ppm (TWA), 150 ppm (STEL) | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) | 4300 mg/kg (rats, orally) | ||
| LCLo (lowest published) | 6125 ppm (rat, 12 hr) 6125 ppm (human, 12 hr) | ||
| NIOSH (US health exposure limits): | |||
| PEL (Permissible) | TWA 100 ppm (435 mg/m) | ||
| REL (Recommended) | TWA 100 ppm (435 mg/m) ST 150 ppm (655 mg/m) | ||
| IDLH (Immediate danger) | 900 ppm | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
| Related aromatic hydrocarbons | m-xylene p-xylene toluene | ||
| Supplementary data page | |||
| O-Xylene (data page) | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
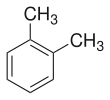
o-Xylene (ortho-xylene) is an aromatic hydrocarbon with the formula C6H4(CH3)2, with two methyl substituents bonded to adjacent carbon atoms of a benzene ring (the ortho configuration). It is a constitutional isomer of m-xylene and p-xylene, the mixture being called xylene or xylenes. o-Xylene is a colourless slightly oily flammable liquid.
Production and use
Petroleum contains about one weight percent xylenes. Most o-xylene is produced by cracking petroleum, which affords a distribution of aromatic compounds, including xylene isomers. m-Xylene is isomerized to o-xylene. Net production was approximately 500,000 tons in the year 2000.
o-Xylene is largely used in the production of phthalic anhydride, which is a precursor to many materials, drugs, and other chemicals. Related to their easy oxidation, the methyl groups are susceptible to halogenation. When treated with elemental bromine, these groups are brominated, yielding xylylene dibromide:
- C6H4(CH3)2 + 2 Br2 → C6H4(CH2Br)2 + 2 HBr
Toxicity and exposure
Xylenes are not acutely toxic, for example the LD50 (rat, oral) is 4300 mg/kg. Effects vary with animal and xylene isomer. Concerns with xylenes focus on narcotic effects.
References
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. 121, 139, 653. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0668". National Institute for Occupational Safety and Health (NIOSH).
- Rudolph, H.D.; Walzer, K.; Krutzik, Irmhild (1973). "Microwave spectrum, barrier for methyl rotation, methyl conformation, and dipole moment of ortho-xylene". Journal of Molecular Spectroscopy. 47 (2): 314. Bibcode:1973JMoSp..47..314R. doi:10.1016/0022-2852(73)90016-7.
- ^ "o-Xylene". International Chemical Safety Cards. ICSC/NIOSH. July 1, 2014.
- O-xylene toxicity
- "Xylene (o-, m-, p-isomers)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Fabri, Jörg; Graeser, Ulrich; Simo, Thomas A. (2000). "Xylenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a28_433. ISBN 978-3527306732.
- Emily F. M. Stephenson (1954). "o-Xylylene Dibromide". Organic Syntheses. 34: 100. doi:10.15227/orgsyn.034.0100.
| Hydrocarbons | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saturated aliphatic hydrocarbons |
| ||||||||||||||||||||||||||||||||||
| Unsaturated aliphatic hydrocarbons |
| ||||||||||||||||||||||||||||||||||
| Aromatic hydrocarbons |
| ||||||||||||||||||||||||||||||||||
| Other | |||||||||||||||||||||||||||||||||||