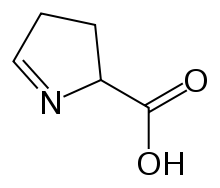
| |
| Names | |
|---|---|
| Preferred IUPAC name 3,4-Dihydro-2H-pyrrole-2-carboxylic acid | |
| Other names
1-Pyrroline-5-carboxylic acid δ-1-Pyrroline-5-carboxylic acid P5C | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
| MeSH | Delta-1-pyrroline-5-carboxylate |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C5H7NO2 |
| Molar mass | 113.115 g/mol |
| Acidity (pKa) | 1.82/6.07 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
1-Pyrroline-5-carboxylic acid (systematic name 3,4-dihydro-2H-pyrrole-2-carboxylic acid) is a cyclic imino acid. Its conjugate base and anion is 1-pyrroline-5-carboxylate (P5C). In solution, P5C is in spontaneous equilibrium with glutamate-5-semialdhyde (GSA).
Biochemistry
The stereoisomer (S)-1-pyrroline-5-carboxylate (also referred to as L-P5C) is an intermediate metabolite in the biosynthesis and degradation of proline and arginine.
In prokaryotic proline biosynthesis, GSA is synthesized from γ-glutamyl phosphate by the enzyme γ-glutamyl phosphate reductase. In most eukaryotes, GSA is synthesised from the amino acid glutamate by the bifunctional enzyme 1-pyrroline-5-carboxylate synthase (P5CS). The human P5CS is encoded by the ALDH18A1 gene. The enzyme pyrroline-5-carboxylate reductase converts P5C into proline.
In proline degradation, the enzyme proline dehydrogenase produces P5C from proline, and the enzyme 1-pyrroline-5-carboxylate dehydrogenase converts GSA to glutamate. In many prokaryotes, proline dehydrogenase and P5C dehydrogenase form a bifunctional enzyme that prevents the release of P5C during proline degradation.
A reciprocal regulation of delta 1-pyrroline-5-carboxylate synthetase (P5CS) and proline dehydrogenase genes controls proline levels during and after osmotic stress in plants proportional to the level of proline. This allows an optimum level of proline to be produced from reduced nitrogen to control osmotic stress.
References
- "computed by Chemicalize from ChemAxon".
- PubChem. "3,4-Dihydro-2H-pyrrole-2-carboxylic acid". pubchem.ncbi.nlm.nih.gov. Retrieved 2020-01-23.
- Heacock, Anne M.; Williams, Irene H.; Frank, Leonard H.; Adams, Elijah (1975-04-01). "Δ1-Pyrroline-5-carboxylate and Δ1-pyrroline-3-hydroxy-5-carboxylate: Chromatography on the amino acid analyzer". Analytical Biochemistry. 64 (2): 593–600. doi:10.1016/0003-2697(75)90472-8. ISSN 0003-2697. PMID 236687.
- Bertolo, Robert F.; Burrin, Douglas G. (2008-10-01). "Comparative Aspects of Tissue Glutamine and Proline Metabolism". The Journal of Nutrition. 138 (10): 2032S–2039S. doi:10.1093/jn/138.10.2032S. ISSN 0022-3166. PMID 18806120.
- Qamar, Aarzoo; Mysore, Kirankumar; Senthil-Kumar, Muthappa (2015). "Role of proline and pyrroline-5-carboxylate metabolism in plant defense against invading pathogens". Frontiers in Plant Science. 6: 503. doi:10.3389/fpls.2015.00503. ISSN 1664-462X. PMC 4491715. PMID 26217357.
- Winter, Gudrun; Todd, Christopher D.; Trovato, Maurizio; Forlani, Giuseppe; Funck, Dietmar (2015). "Physiological implications of arginine metabolism in plants". Frontiers in Plant Science. 6: 534. doi:10.3389/fpls.2015.00534. ISSN 1664-462X. PMC 4520006. PMID 26284079.
- Liu G, Maunoury C, Kamoun P, Aral B (Oct 1996). "Assignment of the human gene encoding the delta 1-pyrroline-5-carboxylate synthetase (P5CS) to 10q24.3 by in situ hybridization". Genomics. 37 (1): 145–6. doi:10.1006/geno.1996.0535. PMID 8921385.
- "Entrez Gene: ALDH18A1 aldehyde dehydrogenase 18 family, member A1".
- Liu, Li-Kai; Becker, Donald F.; Tanner, John J. (2017-10-15). "Structure, function, and mechanism of proline utilization A (PutA)". Archives of Biochemistry and Biophysics. Flavoproteins: Beyond the Classical Paradigms. 632: 142–157. doi:10.1016/j.abb.2017.07.005. ISSN 0003-9861. PMC 5650515. PMID 28712849.
- Verma, D.P.S (December 1996). "Reciprocal regulation of delta 1-pyrroline-5-carboxylate synthetase and proline dehydrogenase genes controls proline levels during and after osmotic stress in plants". Molecular Genetics. 253 (3): 334–341.