| ERTooltip Estrogen receptor | | Agonists |
- Steroidal: 2-Hydroxyestradiol
- 2-Hydroxyestrone
- 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol
- 3α-Androstanediol
- 3α,5α-Dihydrolevonorgestrel
- 3β,5α-Dihydrolevonorgestrel
- 3α-Hydroxytibolone
- 3β-Hydroxytibolone
- 3β-Androstanediol
- 4-Androstenediol
- 4-Androstenedione
- 4-Fluoroestradiol
- 4-Hydroxyestradiol
- 4-Hydroxyestrone
- 4-Methoxyestradiol
- 4-Methoxyestrone
- 5-Androstenediol
- 7-Oxo-DHEA
- 7α-Hydroxy-DHEA
- 7α-Methylestradiol
- 7β-Hydroxyepiandrosterone
- 8,9-Dehydroestradiol
- 8,9-Dehydroestrone
- 8β-VE2
- 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED)
- 11β-Chloromethylestradiol
- 11β-Methoxyestradiol
- 15α-Hydroxyestradiol
- 16-Ketoestradiol
- 16-Ketoestrone
- 16α-Fluoroestradiol
- 16α-Hydroxy-DHEA
- 16α-Hydroxyestrone
- 16α-Iodoestradiol
- 16α-LE2
- 16β-Hydroxyestrone
- 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol)
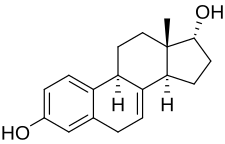
- 17α-Estradiol (alfatradiol)
- 17α-Dihydroequilenin
- 17α-Dihydroequilin
- 17α-Epiestriol (16α-hydroxy-17α-estradiol)
- 17α-Ethynyl-3α-androstanediol
- 17α-Ethynyl-3β-androstanediol
- 17β-Dihydroequilenin
- 17β-Dihydroequilin
- 17β-Methyl-17α-dihydroequilenin
- Abiraterone
- Abiraterone acetate
- Alestramustine
- Almestrone
- Anabolic steroids (e.g., testosterone and esters, methyltestosterone, metandienone (methandrostenolone), nandrolone and esters, many others; via estrogenic metabolites)
- Atrimustine
- Bolandiol
- Bolandiol dipropionate
- Butolame
- Clomestrone
- Cloxestradiol
- Conjugated estriol
- Conjugated estrogens
- Cyclodiol
- Cyclotriol
- DHEA
- DHEA-S
- ent-Estradiol
- Epiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol)
- Epimestrol
- Equilenin
- Equilin
- ERA-63 (ORG-37663)
- Esterified estrogens
- Estetrol
- Estradiol
- Estramustine
- Estramustine phosphate
- Estrapronicate
- Estrazinol
- Estriol
- Estrofurate
- Estrogenic substances
- Estromustine
- Estrone
- Etamestrol (eptamestrol)
- Ethinylandrostenediol
- Ethinylestradiol
- Ethinylestriol
- Ethylestradiol
- Etynodiol
- Etynodiol diacetate
- Hexolame
- Hippulin
- Hydroxyestrone diacetate
- Lynestrenol
- Lynestrenol phenylpropionate
- Mestranol
- Methylestradiol
- Moxestrol
- Mytatrienediol
- Nilestriol
- Norethisterone
- Noretynodrel
- Orestrate
- Pentolame
- Prodiame
- Prolame
- Promestriene
- RU-16117
- Quinestradol
- Quinestrol
- Tibolone
- Xenoestrogens: Anise-related (e.g., anethole, anol, dianethole, dianol, photoanethole)
- Chalconoids (e.g., isoliquiritigenin, phloretin, phlorizin (phloridzin), wedelolactone)
- Coumestans (e.g., coumestrol, psoralidin)
- Flavonoids (incl. 7,8-DHF, 8-prenylnaringenin, apigenin, baicalein, baicalin, biochanin A, calycosin, catechin, daidzein, daidzin, ECG, EGCG, epicatechin, equol, formononetin, glabrene, glabridin, genistein, genistin, glycitein, kaempferol, liquiritigenin, mirificin, myricetin, naringenin, penduletin, pinocembrin, prunetin, puerarin, quercetin, tectoridin, tectorigenin)
- Lavender oil
- Lignans (e.g., enterodiol, enterolactone, nyasol (cis-hinokiresinol))
- Metalloestrogens (e.g., cadmium)
- Pesticides (e.g., alternariol, dieldrin, endosulfan, fenarimol, HPTE, methiocarb, methoxychlor, triclocarban, triclosan)
- Phytosteroids (e.g., digitoxin (digitalis), diosgenin, guggulsterone)
- Phytosterols (e.g., β-sitosterol, campesterol, stigmasterol)
- Resorcylic acid lactones (e.g., zearalanone, α-zearalenol, β-zearalenol, zearalenone, zeranol (α-zearalanol), taleranol (teranol, β-zearalanol))
- Steroid-like (e.g., deoxymiroestrol, miroestrol)
- Stilbenoids (e.g., resveratrol, rhaponticin)
- Synthetic xenoestrogens (e.g., alkylphenols, bisphenols (e.g., BPA, BPF, BPS), DDT, parabens, PBBs, PHBA, phthalates, PCBs)
- Others (e.g., agnuside, rotundifuran)
|
|---|
Mixed
(SERMsTooltip Selective estrogen receptor modulators) |
|
|---|
| Antagonists |
- Coregulator-binding modulators: ERX-11
|
|---|
|
|---|