| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 2,2-Dimethylbutane | |||
| Other names Neohexane, 22DMB | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 1730736 | ||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.825 | ||
| EC Number |
| ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1208 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C6H14 | ||
| Molar mass | 86.178 g·mol | ||
| Appearance | Colorless liquid | ||
| Odor | Odorless | ||
| Density | 649 mg mL | ||
| Melting point | −102 to −98 °C; −152 to −145 °F; 171 to 175 K | ||
| Boiling point | 49.7 to 49.9 °C; 121.4 to 121.7 °F; 322.8 to 323.0 K | ||
| log P | 3.51 | ||
| Vapor pressure | 36.88 kPa (at 20 °C) | ||
| Henry's law constant (kH) |
6.5 nmol Pa kg | ||
| Magnetic susceptibility (χ) | -76.24·10 cm/mol | ||
| Refractive index (nD) | 1.369 | ||
| Thermochemistry | |||
| Heat capacity (C) | 189.67 J K mol | ||
| Std molar entropy (S298) |
272.00 J K mol | ||
| Std enthalpy of formation (ΔfH298) |
−214.4–−212.4 kJ mol | ||
| Std enthalpy of combustion (ΔcH298) |
−4.1494–−4.1476 MJ mol | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |    
| ||
| Signal word | Danger | ||
| Hazard statements | H225, H304, H315, H336, H411 | ||
| Precautionary statements | P210, P261, P273, P301+P310, P331 | ||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | −29 °C (−20 °F; 244 K) | ||
| Autoignition temperature |
425 °C (797 °F; 698 K) | ||
| Explosive limits | 1.2–7.7% | ||
| NIOSH (US health exposure limits): | |||
| PEL (Permissible) | none | ||
| Related compounds | |||
| Related alkanes | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
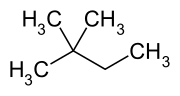
2,2-Dimethylbutane, trivially known as neohexane at William Odling's 1876 suggestion, is an organic compound with formula C6H14 or (H3C-)3-C-CH2-CH3. It is therefore an alkane, indeed the most compact and branched of the hexane isomers — the only one with a quaternary carbon and a butane (C4) backbone.
Synthesis
Butlerov's student V. Goryainov originally discovered neohexane in 1872 by cross-coupling of zinc ethyl with tert-butyl iodide.
2,2-Dimethylbutane can be synthesised by the hydroisomerisation of 2,3-dimethylbutane using an acid catalyst.
It can also be synthesised by isomerization of n-pentane in the presence of a catalyst containing combinations of one or more of palladium, platinum, rhodium and rhenium on a matrix of zeolite, alumina, silicon dioxide or other materials. Such reactions create a mixture of final products including isopentane, n-hexane, 3-methylpentane, 2-methylpentane, 2,3-dimethylbutane and 2,2-dimethylbutane. Since the composition of the final mixture is temperature dependant the desired final component can be obtained choice of catalyst and by combinations of temperature control and distillations.
Uses
Neohexane is used as a high-octane anti-knock additive in gasoline and in the manufacture of agricultural chemicals. It is also used in a number of commercial, automobile and home maintenance products, such as adhesives, electronic contact cleaners and upholstery polish sprays.
In laboratory settings, it is commonly used as a probe molecule in techniques which study the active sites of metal catalysts. Such catalysts are used in hydrogen-deuterium exchange, hydrogenolysis, and isomerization reactions. It is well suited to this purpose as 2,2-dimethylbutane contains both an isobutyl and an ethyl group.
See also
- Methylbutane (isopentane)
- 2-Methylpentane (isohexane)
References
- Haynes, William M. (2010). Handbook of Chemistry and Physics (91 ed.). Boca Raton, Florida, USA: CRC Press. p. 3-194. ISBN 978-1-43982077-3.
- "2,2-DIMETHYLBUTANE - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. Retrieved 9 March 2012.
- NIOSH Pocket Guide to Chemical Hazards. "#0323". National Institute for Occupational Safety and Health (NIOSH).
- Philosophical Magazine. Taylor & Francis. 1876.
- Журнал Русского физико-химического общества (in Russian). Тип-ія Б. Демакова. 1872.
- "2,2-dimethylbutane". National Center for Biotechnology Information. 18 July 2015. Retrieved 20 July 2015.
- Rabo, J. A.; Pickert, P. E.; Mays, R. L. (1961). "Pentane and Hexane Isomerization". Industrial & Engineering Chemistry. 53 (9). American Chemical Society (ACS): 733–736. doi:10.1021/ie50621a029. ISSN 0019-7866.
- Den Hartog, A. J.; Rek, P. J. M.; Botman, M. J. P.; De Vreugd, C.; Ponec, V. (1988). "Reactions of 2,2-dimethylbutane on platinum-rhenium/alumina catalysts. Effect of sulfur and chlorine on the selectivity". Langmuir. 4 (5). American Chemical Society (ACS): 1100–1103. doi:10.1021/la00083a006. ISSN 0743-7463.
- Brown, Ronald; Kemball, Charles; McDougall, Gordon S. (1995). "Exchange reactions of 2,2-dimethylpentane, 2,2-dimethylbutane and 2,2-dimethylpropane over Pt/SiO2 and Rh/SiO2". Journal of the Chemical Society, Faraday Transactions. 91 (7). Royal Society of Chemistry (RSC): 1131. doi:10.1039/ft9959101131. ISSN 0956-5000.
- "Hazardous Substance Fact Sheet - 2,2-Dimethylbutane" (PDF). New Jersey Department of Health. June 2008. Retrieved 2 July 2021.
- "2,2-Dimethylbutane". Consumer Products Information Database. 2021. Retrieved 2 July 2021.
- Burch, R.; Paál, Z. (1994). "The use of 2,2-dimethylbutane (neohexane) as a probe molecule of metal catalysts". Applied Catalysis A: General. 114 (1). Elsevier BV: 9–33. doi:10.1016/0926-860x(94)85106-9. ISSN 0926-860X.

