| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 2,4,6-Trichlorophenol | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 776729 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.633 | ||
| EC Number |
| ||
| Gmelin Reference | 3766 | ||
| KEGG | |||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 2020 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C6H2Cl3OH/C6H3Cl3O | ||
| Molar mass | 197.45 g/mol | ||
| Appearance | yellow-whitish lumps or powder | ||
| Density | 1.4901 g/cm at 75 °C | ||
| Melting point | 69.5 °C (157.1 °F; 342.6 K) | ||
| Boiling point | 249 °C (480 °F; 522 K) | ||
| Solubility in water | 0.069 g/100 g H2O | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |   
| ||
| Signal word | Warning | ||
| Hazard statements | H302, H315, H319, H351, H410 | ||
| Precautionary statements | P201, P202, P264, P270, P273, P280, P281, P301+P312, P302+P352, P305+P351+P338, P308+P313, P321, P330, P332+P313, P337+P313, P362, P391, P405, P501 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
2,4,6-Trichlorophenol, also known as TCP, phenaclor, Dowicide 2S, Dowcide 2S, omal, is a chlorinated phenol that has been used as a fungicide, herbicide, insecticide, antiseptic, defoliant, and glue preservative. It is a clear to yellowish crystalline solid with a strong, phenolic odor. It decomposes on heating to produce toxic and corrosive fumes including hydrogen chloride and chlorine.
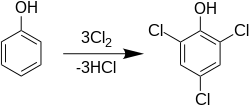
Preparation
2,4,6-Trichlorophenol is produced industrially by the electrophilic chlorination of phenol:
Health effects
In animal models, consumption of 2,4,6-trichlorophenol leads to an increased incidence of lymphomas, leukemia, and liver cancer. It is classified as Group B2 (probable human carcinogen) by the United States Environmental Protection Agency. The technical grade of this substance may contain polychlorinated dibenzodioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), and other contaminants.
Environmental effects
2,4,6-Trichlorophenol is an environmental pollutant that has been found in fresh water lakes such as the Great Lakes.
See also
- Trichlorophenol (for other isomers).
References
- ^ Haynes, p. 3.522
- William M. Haynes, ed. (2016). CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data (97th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4987-5428-6. OCLC 930681942.
- Ogunniyi TA, Oni PO, Juba A, Asaolu SO, Kolawole DO (2000-01-05). "Disinfectants/antiseptics in the management of guinea worm ulcers in the rural areas". Acta Tropica. 74 (1): 33–38(6). doi:10.1016/S0001-706X(99)00057-1. PMID 10643905.
- "Safety data for 2,4,6-trichlorophenol". University of Oxford. 2005-09-05. Archived from the original on 2007-10-14. Retrieved 2007-11-16.
- Muller, François; Caillard, Liliane (2011-10-15), "Chlorophenols", in Wiley-VCH Verlag GmbH & Co. KGaA (ed.), Ullmann's Encyclopedia of Industrial Chemistry, Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, pp. a07_001.pub2, doi:10.1002/14356007.a07_001.pub2, ISBN 978-3-527-30673-2, retrieved 2022-03-13
- "2,4,6-Trichlorophenol". The Carcinogenic Potency Database Project, University of Berkeley. 2007-10-03. Archived from the original on 4 December 2007. Retrieved 2007-11-16.
- ^ "2,4,6 Trichlorophenol". United States Environmental Protection Agency. Jan 2000. Retrieved 2007-11-16.
- "2,4,6-Trichlorophenol". IPCS. Nov 1998. Archived from the original on 2013-06-27. Retrieved 2007-11-16.
- TP Halappa Gowdal; John D Lock; Ruth G Kurtz (Feb 1985). "A comprehensive study of risk assessment for a hazardous compound of public health concern". Water, Air, & Soil Pollution. 24 (2): 189. Bibcode:1985WASP...24..189H. doi:10.1007/BF00285444. S2CID 96067556.
Cited sources
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.