| |
| Names | |
|---|---|
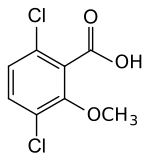
| Preferred IUPAC name 3,6-Dichloro-2-methoxybenzoic acid | |
| Other names
3,6-Dichloro-o-anisic acid Dianat | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.016.033 |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H6Cl2O3 |
| Molar mass | 221.03 g·mol |
| Appearance | White crystalline solid |
| Density | 1.57 |
| Melting point | 114 to 116 °C (237 to 241 °F; 387 to 389 K) |
| Solubility in water | "low" |
| Solubility in acetone | 810 g/L |
| Solubility in ethanol | 922 g/L |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H302, H318, H412 |
| Precautionary statements | P273, P280, P305+P351+P338 |
| Flash point | 199 °C (390 °F; 472 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Dicamba (3,6-dichloro-2-methoxybenzoic acid) is a selective systemic herbicide first registered in 1967. Brand names for formulations of this herbicide include Dianat, Banvel, Diablo, Oracle and Vanquish. This chemical compound is a chlorinated derivative of o-anisic acid. It has been described as a "widely used, low-cost, environmentally friendly herbicide that does not persist in soils and shows little or no toxicity to wildlife and humans."
Despite its success in improving crop yields, dicamba has attracted controversy. According to the United States Environmental Protection Agency (EPA), dicamba's primary ecological risk is for non-target terrestrial plants from exposure through spray drift, whereby dicamba inadvertently migrates to non-targeted neighboring areas, damaging those plants.
In 2016, dicamba was approved for use in the United States over GMO dicamba-resistant crops created by Monsanto. Dicamba came under significant scrutiny due to its tendency to spread from treated fields into neighboring fields, causing damage. The controversy led to litigation, state bans and additional restrictions over dicamba use.
Use as an herbicide

Dicamba is a selective and systemic herbicide that kills annual and perennial broadleaf weeds. Its primary commercial applications are weed control for grain crops and turf areas. It is also used to control brush and bracken in pastures, as well as controlling legumes and cacti. In combination with a phenoxy herbicide or with other herbicides, dicamba can be used for weed control in range land and other noncrop areas (fence rows, roadways, and wastage). Dicamba is toxic to conifer species but is in general less toxic to grasses. Dicamba is a synthetic auxin that functions by increasing plant growth rate, leading to senescence and cell death.
The growth regulating properties of dicamba were first discovered by Zimmerman and Hitchcock in 1942. Soon after, Jealott's Hill Experimental Station in England was evaluating dicamba in the field. Dicamba has since been used for household and commercial weed control.
Increasing use of dicamba has been reported with the release of dicamba-resistant genetically modified plants by Monsanto. In October 2016, the EPA launched a criminal investigation into the illegal application of older, drift prone formulations of dicamba onto these new plants. Older formulations have been reported to drift after application and affect other crops not meant to be treated. A less volatile formulation of dicamba made by Monsanto, designed to be less prone to vaporizing and inhibit unintended drift between fields, was approved for use in the United States by the EPA in 2016, and was commercially available in 2017. As a result, the use of dicamba in US agriculture rose sharply from approximately 8,000,000 pounds (3,600,000 kg) in 2016 to 30,000,000 pounds (14,000,000 kg) in 2019, according to the US Geological Survey.
Drift
Dicamba came under scrutiny due to its reputation for drifting from treated fields onto neighboring crops. In 2011, the European Food Safety Authority identified dicamba's potential for long-range transport through the atmosphere as a critical area of concern. In 2022, the United States Environmental Protection Agency identified spray drift as the primary ecological risk for dicamba due to its potential effects on non-target terrestrial plants. Dicamba is also available in a drift-resistant formulation, which is less likely to affect neighboring fields.
Toxicology
Humans
In 2022 the EPA identified potential occupational risks to handlers mixing and loading dry flowable formulations for application to sod and field crops. The Agency did not identify dietary, residential, aggregate, or post-application risks of concern.
Increased lung and colon cancer rate ratios and positive exposure–response patterns were reported for dicamba, in a review of data gathered in the National Institutes of Health's Agricultural Health Study. The Cross-Canada Study of Pesticides and Health found that exposure to dicamba increased the risk of non-Hodgkins lymphoma in men.
Mammals
Dicamba has low toxicity by ingestion and inhalation or dermal exposure (oral LD50 in rats: 757 mg/kg body weight, dermal LD50 in rats: >2,000 mg/kg, inhalation LC50 in rats: >200 mg/L). In a three-generation study, dicamba did not affect the reproductive capacity of rats.
When rabbits were given doses of 0, 0.5, 1, 3, 10, or 20 (mg/kg)/day of technical dicamba from days 6 through 18 of pregnancy, toxic effects on the mothers, slightly reduced fetal body weights, and increased loss of fetuses occurred at the 10 mg/kg dose. U.S. Environmental Protection Agency (EPA) has set the NOAEL for this study at 3 (mg/kg)/day.
In dog tests, some enlargement of liver cells has occurred, but a similar effect has not been shown in humans.
Aquatic animals
Dicamba and its derivatives are practically nontoxic to aquatic invertebrates. Studies suggest that dicamba should be considered to be a potential endocrine disruptor for fish at environmentally relevant concentrations.
Birds and bees
The 2022 EPA draft ecological risk assessment identified potential adverse effects to birds, and bee larvae for all dicamba uses.
Genetically modified crops
The soil bacterium Pseudomonas maltophilia (strain DI-6) converts dicamba to 3,6-dichlorosalicylic acid (3,6-DCSA), which lacks herbicidal activity. The enzymes responsible for this first breakdown step comprise a three-component system called dicamba O-demethylase.
In the 2000s, Monsanto incorporated one component of the three enzymes into the genome of soybean, cotton, and other broadleaf crop plants, making them resistant to dicamba. Monsanto has marketed their dicamba resistant crops under the brand name Xtend.
Farmers have expressed concern about being forced to grow resistant crops as protection against drifting dicamba.
Resistance
Herbicide resistance after the introduction of resistant crops is a common concern with herbicide. In the laboratory, researchers have demonstrated weed resistance to dicamba within three generations of exposure. This effect is illustrated by glyphosate-resistant crops (marketed as 'Roundup Ready'). The weed species Amaranthus palmeri and Bassia scoparia developed resistance to dicamba already in the 1990s.
Dicamba's HRAC resistance class is Group I (Aus), Group O (Global), Group 4 (numeric).
Environmental fate

Dicamba is poorly soluble in water. A study conducted from 1991 to 1996 by the U.S. Geologic Survey found dicamba in 0.13% of the groundwaters surveyed. The maximum level detected was 0.0021 mg/L.
It is biodegraded by both aerobic and anaerobic bacteria to 3,6-dichlorosalicylic acid. This conversion is catalyzed by a dicamba monooxygenase, which hydroxylates the methyl group of dicamba. The hydroxymethylated derivative hydrolyzes readily to an inactive dichlorosalicylic acid or its conjugate base. Modification of the gene encoding this enzyme is one strategy toward dicamba-resistant crops. The dichlorosalicylic acid is far less toxic than dicamba, which is already rather low.
Use on dicamba-tolerant crops
Complaints against dicamba accelerated after the United States EPA approved a Monsanto-created soybean which could tolerate it in 2016. The soybean was a part of Monsanto's Xtend products. Dicamba was approved by the EPA for "over-the-top" (OTT) use on those dicamba-tolerant soybean and cotton crops. In 2017 and again in 2018, EPA amended the registrations of all OTT dicamba products following reports that farmers had experienced crop damage and economic losses resulting from spray drifting.
Arkansas and Missouri banned the sale and use of dicamba in July 2017 in response to complaints of crop damage due to drift. Monsanto responded by arguing that not all instances of crop damage had been investigated and a ban was premature. Monsanto sued the state of Arkansas to stop the ban, but the case was dismissed in February 2018. It has also been acknowledged that the use of dicamba had increased since 2017.
In June 2020, the 9th U.S. Circuit Court of Appeals blocked sales of three dicamba-based herbicides in the United States, finding that the Environmental Protection Agency "substantially understated risks that it acknowledged and failed entirely to acknowledge other risks." The EPA's Office of the Inspector General concluded that the EPA had deviated from typical procedures in its 2018 decision despite the best efforts of EPA's career scientists and managers to recommend a different approach that was scientifically, procedurally, and legally sound.
On 8 June 2020, the EPA clarified that existing stocks of the dicamba-based pesticides bought before 3 June 2020 may be used according to their previous labels until 31 July 2020. In October 2020 the EPA issued a decision on the registration application of three dicamba-based products, Xtendimax, Engenia, and Tavium. They approved of their use from 2021 to 2025 with some additional changes, including labeling restrictions.
Despite the control measures implemented by the EPA in 2020, the 2021 incident reports showed little change in the number, severity, and/or geographic extent of dicamba-related incidents. In March 2022 and in February 2023, EPA approved additional labeling to further restrict use of OTT dicamba to reduce the likelihood of volatility and offsite movement of OTT dicamba by avoiding application on days with high temperatures. The restriction apply to Minnesota, Iowa, Illinois, Indiana, and South Dakota.
Lawsuits
In February 2018, it was reported that numerous farmers from 21 states had filed lawsuits against Monsanto alleging that dicamba damaged their crops, with the most prominent cases coming from Missouri and Arkansas. By August 2019, more lawsuits were filed, alleging that dicamba had damaged crops, gardens, and trees of neighbors of the farmers who used it.
On 27 January 2020, the first trial concerning dicamba-related products began in Cape Girardeau, Missouri. The lawsuit involves a peach farmer who alleged that dicamba-based herbicides caused significant damage to his crops and trees. It had also been filed in November 2016, when dicamba was still owned by Monsanto. On 14 February 2020, the jury involved in the lawsuit ruled against dicamba owner Bayer and its co-defendant BASF and found in favor of the peach grower, Bader Farms owner Bill Bader. Bayer and BASF were also ordered to pay Bader $15 million in damages. On 15 February 2020, Monsanto and BASF were ordered to pay an additional $250 million in punitive damages. Court documents revealed Monsanto had used dicamba drift as a sales pitch to convince farmers to buy their proprietary dicamba-resistant seeds or face devastated crops.
On 17 February, it was announced that dicamba would face many more lawsuits. On 26 February, the Peiffer Wolf Carr & Kane Law Firm announced that after the Bader verdict, more than 2,000 U.S. farmers hired the law firm to represent them in upcoming lawsuits.
In June 2020, Bayer agreed to a settlement of up to $400 million for all 2015–2020 crop year dicamba claims, not including the $250 million judgement. On 25 November 2020, U.S. District Judge Stephen Limbaugh Jr. reduced the punitive damage amount in the Bader Farms case to $60 million.
References
- Merck Index, 11th Edition, 3026.
- ^ Arnold P. Appleby, Franz Müller. "Weed Control, 2" in Ullmann's Encyclopedia of Industrial Chemistry 2011, Wiley-VCH, Weinheim. doi:10.1002/14356007.o28_o01
- Record of Dicamba in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2023-12-12.
- "Reregistration Eligibility Decision for Dicamba and Associated Salts - US EPA" (PDF). 8 June 2006.
- "Dicamba (Banvel) Herbicide Profile 10/83, Pesticide Management Education Program". Cornell University.
- ^ Behrens, M. R.; Mutlu, N.; Chakraborty, S.; Dumitru, R.; Jiang, W. Z.; Lavallee, B. J.; Herman, P. L.; Clemente, T. E.; Weeks, D. P. (2007). "Dicamba Resistance: Enlarging and Preserving Biotechnology-Based Weed Management Strategies". Science. 316 (5828): 1185–8. Bibcode:2007Sci...316.1185B. doi:10.1126/science.1141596. PMID 17525337. S2CID 7093076.
- "Dicamba". United States Environmental Protection Agency. 16 February 2023.
- ^ "Dicamba". United States Environmental Protection Agency. 16 February 2023. Archived from the original on 23 December 2023.
- Revealed: Monsanto predicted crop system would damage US farms The Guardian, 2020
- "Dicamba (Ref: SAN 837H)". Pesticide Properties DataBase. University of Hertfordshire.
- Gleason, Cynthia; Foley, Rhonda C.; Singh, Karam B. (2011). "Mutant Analysis in Arabidopsis Provides Insight into the Molecular Mode of Action of the Auxinic Herbicide Dicamba". PLOS ONE. 6 (3): e17245. Bibcode:2011PLoSO...617245G. doi:10.1371/journal.pone.0017245. PMC 3050828. PMID 21408147.
- Peterson, Gale E. (1967). "The Discovery and Development of 2,4-D". Agricultural History. 41 (3). Agricultural History Society: 243–254. JSTOR 3740338. Retrieved 10 November 2020.
- Weinraub, Mark (25 October 2016). "U.S. agency searches for proof of criminal use of herbicide dicamba". Reuters. Retrieved 29 October 2016.
- "EPA Probes Dicamba Use- Federal Search Warrants Issued in Missouri". KTIC Radio. 25 October 2016. Retrieved 4 October 2017.
- Gray, Bryce (5 August 2016). "Suspected illegal herbicide use takes toll on southeast Missouri farmers". St Louis Post-Dispatch. Retrieved 16 August 2016.
- Gray, Bryce (14 August 2016). "Illegal herbicide use may threaten survival of Missouri's largest peach farm". St Louis Post-Dispatch. Retrieved 16 August 2016.
- Gray, Bryce (9 November 2016). "EPA approves Monsanto's less-volatile form of dicamba herbicide". St. Louis Post-Dispatch. Retrieved 24 June 2017.
- US Geological Survey (12 October 2021). "Estimated Agricultural Use for Dicamba, 2019". Retrieved 27 December 2021.
- "Dicamba Know-How: Seven Things to Know Before You Spray Dicamba in 2020". Progressive Farmer. Retrieved 10 July 2020.
- Pollack, Andrew (25 April 2012). "Dow Corn, Resistant to a Weed Killer, Runs into Opposition". The New York Times. Archived from the original on 4 January 2013. Retrieved 1 August 2016.
- "Conclusion on the peer review of the pesticide risk assessment of the active substance dicamba | EFSA". www.efsa.europa.eu. 14 January 2011. doi:10.2903/j.efsa.2011.1965. Retrieved 23 December 2023.
- Weichenthal, Scott; Moase, Connie; Chan, Peter (August 2010). "A Review of Pesticide Exposure and Cancer Incidence in the Agricultural Health Study Cohort". Environmental Health Perspectives. 118 (8): 1117–1125. doi:10.1289/ehp.0901731. PMC 2920083.
- McDuffie, Helen H.; Pahwa, Punam; McLaughlin, John R.; Spinelli, John J.; Fincham, Shirley; Dosman, James A.; Robson, Diane; Skinnider, Leo F.; Choi, Norman W. (November 2001). "Non-Hodgkin's Lymphoma and Specific Pesticide Exposures in Men". Cancer Epidemiology, Biomarkers & Prevention. 10 (11). Retrieved 13 April 2021.
- ^ "Pesticide Information Profile – Dicamba". Pesticide Management Education Program, Cornell University. September 1993. Archived from the original on 27 September 2020.
- Zhu, L; Li, W; Zha, J; Wang, Z (2015). "Dicamba affects sex steroid hormone level and mRNA expression of related genes in adult rare minnow (Gobiocypris rarus) at environmentally relevant concentrations". Environmental Toxicology. 30 (6): 693–703. Bibcode:2015EnTox..30..693Z. doi:10.1002/tox.21947. PMID 24420721. S2CID 45373092.
- ^ Charles, Dan (1 August 2016). "Crime in the Fields: How Monsanto And Scofflaw Farmers Hurt Soybeans in Arkansas". NPR. Retrieved 1 August 2016.
- ^ "Business: Monsanto's Superweeds Saga Is Only Getting Worse". Yahoo, TakePart.com. 2 August 2016. Retrieved 3 August 2016.
- The Rise of Superweeds scientificamerican.com
- Cranston, Harwood J.; Kern, Anthony J.; Hackett, Josette L.; Miller, Erica K.; Maxwell, Bruce D.; Dyer, William E. (2001). "Dicamba resistance in kochia". Weed Science. 49 (2): 164. doi:10.1614/0043-1745(2001)049[0164:DRIK]2.0.CO;2.
- "Australia Herbicide Classification Lookup". Herbicide Resistance Action Committee.
- "2024 HRAC GLOBAL HERBICIDE MOA CLASSIFICATION MASTER LIST". Herbicide Resistance Action Committee.
- Kolpin, Dana W.; Barbash, Jack E.; Gilliom, Robert J. (November 2000). "Pesticides in Ground Water of the United States, 1992–1996". Groundwater. 38 (6): 858–863. Bibcode:2000GrWat..38..858K. doi:10.1111/j.1745-6584.2000.tb00684.x.
- Dumitru, Razvan; Jiang, Wen Zhi; Weeks, Donald P.; Wilson, Mark A. (2009). "Crystal Structure of Dicamba Monooxygenase: A Rieske Nonheme Oxygenase that Catalyzes Oxidative Demethylation". Journal of Molecular Biology. 392 (2): 498–510. doi:10.1016/j.jmb.2009.07.021. PMC 3109874. PMID 19616011.
- Krueger, James P.; Butz, Robert G.; Atallah, Yousef H.; Cork, Douglas J. (1989). "Isolation and identification of microorganisms for the degradation of Dicamba". Journal of Agricultural and Food Chemistry. 37 (2): 534–538. doi:10.1021/jf00086a057. ISSN 0021-8561.
- Milligan, Peter W.; Häggblom, Max M. (1 April 1999). "Biodegradation and Biotransformation of Dicamba under Different Reducing Conditions". Environmental Science & Technology. 33 (8): 1224–1229. Bibcode:1999EnST...33.1224M. doi:10.1021/es981117e. ISSN 0013-936X.
- ^ "Dicamba Lawsuit | Crop Damage Compensation for Farmers". ConsumerNotice.org.
- Gray, Bryce (7 July 2017). "Missouri and Arkansas ban dicamba herbicide as complaints snowball". St. Louis Post-Dispatch. Retrieved 10 July 2017.
- Pucci, Jackie (10 July 2017). "Monsanto Responds to Arkansas, Missouri Dicamba Bans". Crop Life. Retrieved 10 July 2017.
- Nosowitz, Dan (20 February 2018). "Monsanto's Lawsuit Against Arkansas for Dicamba Ban Dismissed". Modern Farmer. Retrieved 7 March 2018.
- ^ "Despite Federal, State Efforts, Dicamba Complaints Continue". Illinois Public Media. 31 August 2019.
- ^ "Farmers, Conservationists Challenge Approval of Monsanto's Crop-damaging Dicamba Pesticide". Center for Biological Diversity.
- "U.S. Court Blocks Sales of Bayer Weed Killer in United States". Reuters. 4 June 2020. Retrieved 11 June 2020.
- US EPA, OCSPP (8 June 2020). "Final Cancellation Order for Three Dicamba Products". US EPA. Retrieved 11 June 2020.
- "EPA Offers Clarity to Farmers in Light of Recent Court Vacatur of Dicamba Registrations". EPA. 8 June 2020. Retrieved 11 June 2020.
- "EPA Approves Dicamba Pesticides Through 2025; Additional Restrictions Imposed". Texas Agricultural Extension. 9 November 2020. Retrieved 26 May 2021.
- "Dicamba Damage Lawsuit - Monsanto Herbicide Lawyers". ClassAction.com.
- Ruff, Corinne (28 January 2020). "Dicamba-Related Federal Trial Begins in Southeast Missouri". news.stlpublicradio.org.
- "Bayer/BASF-Dicamba Lawsuit | KDUZ".
- Reporting, Johnathan Hettinger Midwest Center for Investigative. "Monsanto's defense: Fungal disease, not dicamba, to blame for peach farmer's problems". stltoday.com.
- Reporting, Johnathan Hettinger Midwest Center for Investigative. "Dicamba on trial: Monsanto officials limited testing on their own plots". stltoday.com.
- Ruff, Corinne (24 January 2020). "Dicamba Goes on Trial: The History Behind Monsanto's Friendship-Wilting Weed Killer". news.stlpublicradio.org.
- Reporting, Johnathan Hettinger Midwest Center for Investigative. "Peach farmer takes stand in lawsuit against Bayer, BASF". stltoday.com.
- Gray, Bryce. "Jury finds in favor of Missouri peach grower in lawsuit against Bayer, BASF". stltoday.com.
- "Bayer's Dicamba Hit Tests Patience of Frustrated Investors". Bloomberg.com. 14 February 2020.
- Ruff, Corinne (15 February 2020). "Monsanto, BASF Will Pay $250 Million in Punitive Damages in First Dicamba Trial". news.stlpublicradio.org.
- "Missouri Farm Awarded $265M in Suit Against BASF and Bayer". The New York Times. Associated Press. 15 February 2020.
- McGlashen, Andy (Winter 2022). "Bitter Harvest". Audubon. New York, NY: Audubon Society: 45. ISSN 0004-7694. OCLC 6823366.
- Feeley, Jef; Bross, Tim; Bloomberg (17 February 2020). "Bayer is facing a new wave of herbicide lawsuits—and this time it's not over Monsanto's Roundup". fortune.com. Retrieved 17 December 2020.
- "More Than 2,000 Farmer Are Expected To File Dicamba-Damage Lawsuits, Says Law Firm". Successful Farming. 26 February 2020. Retrieved 11 March 2020.
- Bayer To Pay More Than $10 Billion To Resolve Cancer Lawsuits Over Weedkiller Roundup
- Reeves, J.C. (15 December 2020). "District Judge orders reduction of punitive damages in dicamba case". Southeast Missourian. Retrieved 15 December 2020.
External links
- Appendix E: Herbicide Information, US Department of Agriculture
- Chemical Fact Sheet, Speclab.com
- Dicamba Technical Fact Sheet – National Pesticide Information Center
- Dicamba General Fact Sheet – National Pesticide Information Center
- Dicamba Pesticide Information Profile – Extension Toxicology Network
- Monsanto's Xtend Crop System Product Page
- EPA Dicamba Reregistration Eligibility Decision