| |
| Names | |
|---|---|
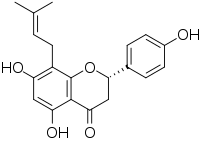
| IUPAC name (2S)-4′,5,7-Trihydroxy-8-(3-methylbut-2-en-1-yl)flavan-4-one | |
| Systematic IUPAC name (2S)-5,7-Dihydroxy-2-(4-hydroxyphenyl)-8-(3-methylbut-2-en-1-yl)-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names Hopein; Flavaprenin; Sophoraflavanone B | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C20H20O5 |
| Molar mass | 340.375 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
8-Prenylnaringenin (8-PN; also known as flavaprenin, (S)-8-dimethylallylnaringenin, hopein, or sophoraflavanone B) is a prenylflavonoid phytoestrogen. It is reported to be the most estrogenic phytoestrogen known. The compound is equipotent at the two forms of estrogen receptors, ERα and ERβ, and it acts as a full agonist of ERα. Its effects are similar to those of estradiol, but it is considerably less potent in comparison.
8-PN is found in hops (Humulus lupulus) and in beer, and is responsible for the estrogenic effects of the former. It can be produced from isoxanthohumol in fungal cells cultures, and by flora in the human intestine.
Properties
Estrogenic
8-PN was shown to preserve bone density and has been demonstrated to reduce hot flashes. 8-PN also induces the secretion of prolactin, and increases other estrogenic responses. The compound binds to and activates ERα more times than it does to ERβ.
This prenylflavanoid has drawn interest in the study of hormone replacement therapy, and it is comparable to some selective estrogen-receptor modulators.
In an in vivo study, 8-PN has activated proliferation of mammary cells. At the concentration found in beer, it is unlikely to have an estrogenic effect in breast tissue. Similar to other estrogens, 8-PN induces the expression of the progesterone receptor in various tissues.
Luteinizing hormone (LH) and follicle stimulating hormone (FSH) are suppressed by 8-PN, indicating that it possesses antigonadotropic properties. 8-PN adversely affects male sperm. The role 8-PN plays in fertility requires further research.
Other
In an in vitro study, 8-PN and synthetic derivatives demonstrated anticancer properties. More recently, a radioligand binding study showed enhancements in GABAA receptor activity by 8-PN
Prenylflavonoids from hops, including 8-PN, are ingredients in some breast enlargement supplements, though there is no evidence of its effectiveness for this purpose.
Chemistry
The enzyme naringenin 8-dimethylallyltransferase uses dimethylallyl diphosphate and (−)-(2S)-naringenin to produce diphosphate and sophoraflavanone B (8-prenylnaringenin).
The enzyme 8-dimethylallylnaringenin 2'-hydroxylase uses sophoraflavanone B (8-prenylnaringenin), NADPH, H and O2 to produce leachianone G, NADP and H2O.
Synthesized derivatives of 8-PN are: 7,4′-di-O-methyl-8-prenylnaringenin; 7-O-pentyl-8-prenylnaringenin; 7,4′-Di-O-allyl-8-prenylnaringenin; 7,4′-Di-O-acetyl-8-prenylnaringenin; and 7,4′-Di-O-palmitoyl-8-prenylnaringenin.
8-Neopentylnaringenin and 8-n-heptylnaringenin are synthetic derivatives of 8-PN.
Etymology
There is another compound, 8-isopentenylnaringenin, also known as sophoraflavanone B, from Sophora flavescens, that could properly be called 8-prenylnaringenin by scientific naming convention.
References
- ^ Keiler AM, Zierau O, Kretzschmar G (2013). "Hop extracts and hop substances in treatment of menopausal complaints". Planta Med. 79 (7): 576–9. doi:10.1055/s-0032-1328330. PMID 23512496.
- ^ Hajirahimkhan A, Dietz BM, Bolton JL (2013). "Botanical modulation of menopausal symptoms: mechanisms of action?". Planta Med. 79 (7): 538–53. doi:10.1055/s-0032-1328187. PMC 3800090. PMID 23408273.
- Green SE (2015), In Vitro Comparison of Estrogenic Activities of Popular Women's Health Botanicals (thesis), archived from the original on 2016-02-22, retrieved 2016-01-01
- Nikolic D, Li Y, Chadwick LR, Grubjesic S, Schwab P, Metz P, Van Breemen RB (2004). "Metabolism of 8-prenylnaringenin, a potent phytoestrogen from hops (Humulus lupulus), by human liver microsomes". Drug Metabolism and Disposition. 32 (2): 272–9. doi:10.1124/dmd.32.2.272. PMID 14744951.
- Fu ML, Wang W, Chen F, Dong YC, Liu Xj, Ni H, Chen Qh (2011). "Production of 8-Prenylnaringenin from Isoxanthohumol through Biotransformation by Fungi Cells". Journal of Agricultural and Food Chemistry. 59 (13): 7419–26. doi:10.1021/jf2011722. PMID 21634799.
- Possemiers S, Bolca S, Grootaert C, Heyerick A, Decroos K, Dhooge W, De Keukeleire D, Rabot S, Verstraete W, Van de Wiele T (July 2006). "The Prenylflavonoid Isoxanthohumol from Hops (Humulus lupulus L.) Is Activated into the Potent Phytoestrogen 8-Prenylnaringenin In Vitro and in the Human Intestine". Journal of Nutrition. 136 (7). American Society for Nutrition: 1862–1867. doi:10.1093/jn/136.7.1862. PMID 16772450.
- Bowe, James (November 15, 2012). "The hop phytoestrogen, 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes". Journal of Endocrinology. 191 (2): 399–405. doi:10.1677/joe.1.06919. PMC 1635969. PMID 17088409.
- ^ Overk CR, Guo J, Chadwick LR, Lantvit DD, Minassi A, Appendino G, Chen SN, Lankin DC, Farnsworth NR, Pauli GF, Van Breemen RB, Bolton JL (2008). "In vivo estrogenic comparisons of Trifolium pratense (red clover) Humulus lupulus (hops), and the pure compounds isoxanthohumol and 8-prenylnaringenin". Chemico-Biological Interactions. 176 (1): 30–39. Bibcode:2008CBI...176...30O. doi:10.1016/j.cbi.2008.06.005. PMC 2574795. PMID 18619951.
- Overk CR, Yao P, Chadwick LR, Nikolic D, Sun Y, Cuendet MA, Deng Y, Hedayat AS, Pauli GF, Farnsworth NR, van Breemen RB, Bolton JL (August 2005). "Comparison of the In Vitro Estrogenic Activities of Compounds from Hops (Humulus lupulus) and Red Clover (Trifolium pratense)". J Agric Food Chem. 53 (16): 6246–6253. doi:10.1021/jf050448p. PMC 1815392. PMID 16076101.
- Rad, Hümpel, Schaefer, Schoemaker, Schleuning, Cohen, Burggraaf (September 1, 2006). "Pharmacokinetics and systemic endocrine effects of the phyto-oestrogen 8-prenylnaringenin after single oral doses to postmenopausal women". British Journal of Clinical Pharmacology. 62 (3): 288–296. doi:10.1111/j.1365-2125.2006.02656.x. PMC 1885137. PMID 16934044.
- Bowe (November 2006). "The hop phytoestrogen, 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes". Journal of Endocrinology. 191 (2): 399–405. doi:10.1677/joe.1.06919. PMC 1635969. PMID 17088409.
- Bolca S, Li J, Nikolic D, Roche N, Blondeel P, Possemiers S, De Keukeleire D, Bracke M, Heyerick A, Van Breemen RB, Depypere H (2010). "Disposition of hop prenylflavonoids in human breast tissue". Molecular Nutrition & Food Research. 54 (2): S284–94. doi:10.1002/mnfr.200900519. PMC 3856213. PMID 20486208.
- "Environmental 'hormones' wreck sperm". BBC News. July 2, 2002. Retrieved 2013-06-26.
- ^ Anioł, Mirosław (January 7, 2012). "Antiproliferative activity and synthesis of 8-prenylnaringenin derivatives by demethylation of 7-O- and 4′-O-substituted isoxanthohumols". Med Chem Res. 21 (12): 4230–4238. doi:10.1007/s00044-011-9967-8. PMC 3474914. PMID 23087590.
- Benkherouf AY, Soini SL, Stompor M, Uusi-Oukari M (February 2019). "Positive allosteric modulation of native and recombinant GABAA receptors by hops prenylflavonoids". European Journal of Pharmacology. 852: 34–41. doi:10.1016/j.ejphar.2019.02.034. ISSN 0014-2999. PMID 30797788. S2CID 73456325.
- S. R. Milligan, J. C. Kalita, V. Pocock, V. Van De Kauter, J. F. Stevens, M. L. Deinzer, H. Rong, D. De Keukeleire (December 2000). "The Endocrine Activities of 8-Prenylnaringenin and Related Hop (Humulus lupulus L.) Flavonoids". Journal of Clinical Endocrinology & Metabolism. 85 (12): 4912–4915. doi:10.1210/jcem.85.12.7168. PMID 11134162.
- Chalfoun C, McDaniel C, Motarjem P, Evans GR, Plastic Surgery Educational Foundation DATA Committee (2004). "Breast-Enhancing Pills: Myth and Reality". Plastic and Reconstructive Surgery. 114 (5): 1330–3. doi:10.1097/01.PRS.0000141495.14284.8B. PMID 15457059.
- Breen L, Sugden D, Heyerick A, O'Byrne K, Milligan S (2009). "The effect of synthetic analogues of the phyto-oestrogen 8-prenylnaringenin on tail skin temperature in a rat hot flush model". Proceedings of the Physiological Society. Archived from the original on 3 December 2013.
- Chadwick, Pauli, Farnsworth (July 1, 2005). "The pharmacognosy of Humulus lupulus L. (hops) with an emphasis on estrogenic properties". Phytomedicine. 13 (1–2): 119–31. doi:10.1016/j.phymed.2004.07.006. PMC 1852439. PMID 16360942.
| Estrogen receptor modulators | |||||||
|---|---|---|---|---|---|---|---|
| ERTooltip Estrogen receptor |
| ||||||
| GPERTooltip G protein-coupled estrogen receptor |
| ||||||
| Flavanones and their glycosides | |
|---|---|
| Flavanones | |
| O-methylated flavanones |
|
| C-methylated flavanones | |
| Glycosides |
|
| Acetylated | |
| Acetylated glycosides | |